A Review on Advanced Oxidation Processes for Effective Water Treatment
Nirmalendu Sekhar Mishra 1
*
 , Rajesh Reddy 1
, Rajesh Reddy 1
 , Aneek Kuila 1
, Aneek Kuila 1
 , Ankita Rani 1
, Ankita Rani 1
 , Priya Mukherjee 1
, Priya Mukherjee 1
 , Ahmad Nawaz 1
, Ahmad Nawaz 1
 and Saravanan Pichiah 1
and Saravanan Pichiah 1

DOI: http://dx.doi.org/10.12944/CWE.12.3.02
Copy the following to cite this article:
Mishra N. S, Reddy R, Kuila A, Rani A, Mukherjee P, Nawaz A, Pichiah S. A Review on Advanced Oxidation Processes for Effective Water Treatment. Curr World Environ 2017;12(3). DOI:http://dx.doi.org/10.12944/CWE.12.3.02
Copy the following to cite this URL:
Mishra N. S, Reddy R, Kuila A, Rani A, Mukherjee P, Nawaz A, Pichiah S. A Review on Advanced Oxidation Processes for Effective Water Treatment. Curr World Environ 2017;12(3). Available from: http://www.cwejournal.org/?p=1054
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2017-11-24 |
|---|---|
| Accepted: | 2017-12-16 |
Introduction
The availability of the earth’s fresh water resources has drastically depleted or contaminated due to improper water management, leading to the vulnerable situation. On the other hand the demand for safe potable water is increasing day-by-day due to the exponential growth of population and inability of the traditional treatment.1 There are many traditional and contemporary purification techniques available or practiced for delivering safe water as per the local standards and these spans from basic filtration, adsorption to most advanced techniques; membrane separation and advanced oxidation processes (AOPs). Amongst the contemporary, the latter one is recognized as highly effective in delivery of safe drinking water free of organics, inorganics, and microbes.2,3 Owing to strong reactive oxygen species generation ability of AOPs it was considered for treating different types of water and wastewaters containing the various classification of pollutants including endocrine disrupting chemicals (EDCs), persistent organic pollutants (POPs), total organic carbon (TOC) and micropollutants.4,5 This AOPs is a broad classification wherein consists of various techniques for the generation of reactive oxygen species and is shown in Figure 1. In general, the realistic aim of any water purification techniques is to render water that is free of toxic matters (organic, inorganic and biological). In this regard, AOPs are characterized as best water treatment/purification processes that involve generation of hydroxyl radical (*OH) in sufficient quantity to affect water purification at standard temperature and pressure.6 The significant advantage of AOPs over all existing chemical and biological processes is that they are totally “environmental-friendly” as they neither transfer pollutants from one phase to the other (as in chemical precipitation and adsorption) nor produce massive amounts of hazardous sludge.7-10 The first AOP based water purification/treatment in full scale was proposed in early 1980s and followed by considerable achievements have been reported.11,12 Thus, the present review will emphasize on the various aspects of AOPs for efficient water management.
 |
|
The enhanced degradation of various categories of pollutants by different AOPs has drawn attention from various research communities. The AOP allows the in-situ generation of various reactive oxygen species (ROS) via different process such as sonolysis, ozonation, UV, Fenton processes, etc. These ROS are subsequently utilized towards the degradation of the various categories of pollutants. The insights on the process parameters and degradation of different AOPs have been conferred. Thus, the present article consolidates the significant works that was reported by the various researchers for efficient water management.
Sonolysis
It involves efficient utilization of ultrasonic sound with a frequency range of 20 KHz -10 MHz.13,14 It does not utilize any hazardous chemicals as mediator’s and hence regarded as eco friendly process. The organic molecules are degraded as a result of explosions of the cavitation bubbles formed as a result of ultrasonic/hydrodynamics. The generated cavitation bubbles tend to fluctuate in their size until get collapsed at their resonance size, leads to dissipation of the stored energy causing the explosions.13 The formation of these cavitation bubbles highly dependents on the ultrasound frequency.15 In general, lower frequencies leads to formation of lesser concentration of active bubbles due to presence of high water vapor content in the collapsing bubbles.15 However, high frequency leads to collision among the active bubbles which generates smaller number of reactive species leading to lesser efficiency.15 The schematic of working principle of Sonolysis is depicted in figure 2.
The explosion of these bubbles leads to degradation of the pollutants via pyrolysis at extreme pressure and temperature (500–10,000 atm & 3,000– 5000 K respectively)16,17 subsequently leading to the generation of *OH by the dissociation of water molecules. Thus formed *OH further reacts with the pollutants leading to their simplest form.18,19 The degradation at the bubble-liquid interface is dominated by the *OH. Also, the migration of free radicals from the bubble–liquid interface into the bulk liquid leads to secondary reactions in it. It is facile sludge free process with no additional generation of secondary pollutants, promoting it as one of the most preferred techniques over photolysis, photo-Fenton and Fenton process, etc.14 Moreover, it has inherent ability to treat cloudy water and leads to the efficient degradation of volatile and sparingly soluble organic matters causing high turbidity.19 Nevertheless, its lower viability due to higher energy consumption and lesser mineralization efficiency limitations directs towards intensifying with various other AOPs like photocatalysis (sono-photocatalysis), photo-fenton (sono-fenton), ozonolysis (sono-ozonation), sonophotolysis, etc.
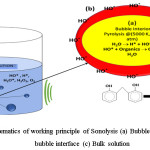 |
|
The mineralization efficiency can also be enhanced by varying the operational parameters such as initial substrate concentration, pH, catalyst loading and ultrasonic power.20 Among the intensified, sono-photolysis involves the synergistic effects of UV irradiation and ultrasound waves towards the mineralization of the pollutants in the absence of catalysts. Whereas, sono-photocatalysis involves the use of UV irradiation and ultrasound waves in the presence of a semiconductor photocatalyst. The synergistic effect of UV/ultrasonic waves leads to enhanced generation of the reactive radicals leading to improved mineralization efficiency. The enhanced generation was attributed to the formation of electron-hole pair as a result of excitation in the semiconductor photocatalyst.21 Additionally, presence of photocatalyst improves the bubble cavitation phenomenon resulting in enhanced migration of the reactive species towards the liquid bulk region.21 Thus, intensification of the above process leads to increased concentration of the free radicals as a result of ultrasound on the peroxide species.22 In case of sono-ozonolysis, the mass transfer of the *OH and ozone are enhanced leading to in-situ generation of H2O2/HO* (each O3 molecules degrades generating 2*OH)22 resulting effective mineralization of the pollutants. The reactive species are generated as a consequence of cavitation phenomenon in water and thermolysis of ozone. The mineralization can be further enhanced either by increasing the pressure or adding peroxide.23 The combination helps in achieving efficiency levels > 80% within a pH range of 5.5 – 6.5.24
Ozonation
Ozone is categorized as strong oxidant and a powerful disinfectant25 after fluorine. Theoretically ozone has been reported to oxidize both inorganic and organic pollutants but practically ozone is highly selective towards pollutants.25 Hence ozone is considered as an electrophile with high selectivity in its reaction.26 Ozonation for water treatment is highly applicable for degrading microbes,26 decolorization,27,28 micropollutants removal,29,30 non-protonated amines26 and taste and odor removal.26,31 This technique is commercially implemented in European countries like Switzerland, Germany and etc as an oxidizer and in wastewater treatment plants.32 Ozone generator, cooling system for ozone generator, pre-treatment unit for dehydrating the air added into the Ozonator and post-treatment reactor for removing excess ozone in the off-gas are the units required for implementation of ozonation.33 The schematic of a typical ozone reactor is shown in figure 3.
![Figure 3: A typical reactor assembly for ozonation. (A=Liquid circulation pump; B=Ozone liquid analyzer; C=Ozone gas analyzer) [34].](http://www.cwejournal.org/wp-content/uploads/2017/12/Vol12_No3_rev_Nir_Fig3-150x150.jpg) |
|
Aquatic phase reaction of Ozone
In water, ozone is wobbly and hence undertakes reaction with the elements of water components.25 The ozone decomposition mechanism in water entangle complex sequence of atoms and single electron transport with intermediate *OH formation and further involves the formation of OH-, HO2, O2-, O3-, HO3, OH, O2 and HO4 entities as shown in figure 4. This ozone decomposition process can be accelerated by escalating the pH or hydrogen peroxide concentration.26 It is well known that Ozone is the predominant disinfectant in water while OH and O3 both act as oxidants in the oxidation process in water with O3 being highly selective and *OH is being highly reactive.26 Hence ozonation can simultaneously be used for disinfection and oxidation. In the inception ozone concentration in water decreases rapidly and then follows first-order kinetics while in the second phase of ozone decrease through oxidation following second-order kinetics.26,35,36
![Figure 4: Ozonation reaction in water matrix [25]](http://www.cwejournal.org/wp-content/uploads/2017/12/Vol12_No3_rev_Nir_Fig4-150x150.jpg) |
|
The half-life of dissolved ozone varies from seconds to hours depending upon the water26,28,37 pH, alkalinity, natural organic matter content.26,28 Among the three the natural organic matter can react with ozone or scavenge *OH i.e. directly or indirectly affecting ozone stability.26 It has been reported that ozone is potent to react with substances directly or indirectly with *OH even at neutral pH as hydrogen peroxide, superoxide ions.25 Ozone, *OH or the blend of the two and subsumed throughout the ozonation of organic and inorganic compounds. Ozone oxidation is enhanced by electron donating groups like –CH3, O-, -OCH326,32 and is reduced by electron withdrawing groups like –Cl, -NO2.26 Sulfidic groups and compounds containing amino groups and double bond also exhibit high ozone reactivity while the reactivity with the saturated compounds is low being accumulated during the ozonation process.26 Low reactivity is also observed in the reaction of ozone with methyl and ethyl ether. Two electron oxidation liberating oxygen atom from ozone is followed in ozonation for oxidizing inorganic compounds.26,38,39 The oxidation potential decreases with protonation of species due to decrease in nucleophilicity.26 Hence the Ozonation can be effectively applied to varieties of pollutants as shown in the Table 1.
Table 1: Various categories of pollutants degraded by ozonation
|
S.No. |
Category |
Pollutant |
Ref. |
|
1) |
Microbial (cyanobacteria) product |
Microcystin-LR |
[40] |
|
2) |
Pesticide |
Carbofuran |
[41] |
|
Dinoseb |
|||
|
3) |
Solvent |
Vinyl chloride |
[26, 42] |
|
Dichloroethenes |
|||
|
4) |
Ligands |
Nitrilotriacetate (NTA) |
[43] |
|
Ethylenediaminetetraacetate (EDTA) |
|||
|
5) |
Pharmaceutical |
Dichlorofenac |
[44] |
|
Carbamazepine |
|||
|
Benzafibrate |
|||
|
Diclofenac |
|||
|
Ibuprofen |
|||
|
Sulfamethoxazole |
|||
|
Roxithromycin |
|||
|
Iopromide |
|||
|
17 α - ethinyl estradiol |
|||
|
6) |
Olefins |
[42] |
|
|
7) |
Deproteinated amines |
[45] |
|
|
8) |
Inorganic micropollutants
|
Fe(II) |
[46] |
|
CN- |
|||
|
Mn(II) |
|||
|
H2S |
|||
|
NO2- |
Influence of process conditions
Higher dosage of ozone at elevated pH with low bromide concentration increases the abatement efficiency of micro-pollutants.29 While low alkalinity and low dissolved oxygen concentrations decrease the oxidation capacity of the system.26 For active compounds like diclofenac, sulfamethoxazole, carbamazepine, trimethoprim, hydrochlorothiazide, phenazone, tramadol, metoprolol etc. with ozone and *OH are independent of pH change without variation in degradation efficiency; however elevated pH readily converts O3 resulting in the abatement of ozone-resistant.29
Limitation of Ozonation
Low oxidation by ozone is reported for chlorinated benzenes, geosmin, and methylisoborneol (MIB), trihalomethanes (THMs) etc.25 Cryptosporidium paravum oocyst are a resistant microbe against disinfection requiring higher amount ozone exposure and hence forms undesirable by-products in drinking water.26,47 Ammonia removal by ozone is slow as it possesses weak oxidation potential.26 It has been seen that the oxidation products produced by ozonation can have unknown toxic matters however these are in low concentration as compared to parental compounds having negligible antimicrobial and estrogenic activities. The toxic matter includes formaldehyde, ketones, phenols, nitromethanes and carcinogens like bromates, N-nitrodimethylamine (NDMA).32,48 Bromate a potential carcinogen formation occurs when ozone and *OH react with bromide in the liquid phase. Apart from all the high costs of ozone as a reagent is a major limiting factor in implementing ozonation.
O3/H2O2
Ozone combined with hydrogen peroxide has emerged as a new dimension in water treatment which can oxidize both inorganic and organic substances more effectively than standalone process. The first studies for wastewater treatment by using O3/H2O2 were performed by simultaneous by Nakayama et. al., and Hango et.al. While, Brunet et.al. and Duguet et.al., performed for treating drinking water.51 Studies showed that H2O2 application in ozonation enhanced the organic substance and Trihalomethanes (THM) precursor oxidation. Its addition increases the ozone transfer rate,51,52-56 follows a single electron transfer forming HO2- which initiates the ozone decomposition cycle forming *OH. The H2O2 incorporation in ozonation especially increases the color removing efficiency but treating drinking water, the oxidation of pesticides, aromatic compounds, and chlorinated solvents mainly applies O3/H2O2,50,61 Benefits of O3/H2O2 usage include shorter reaction time, allowing higher application of ozone doses and low agglomeration of ozone at the reactor outlet. Process conditions like reaction time increment after ozone addition, intensifying pH and applying hydrogen peroxide can enhance the oxidation property of ozone. However, increase in reaction time and pH is not economically viable however H2O2 being a low-cost reagent most commonly applied in drinking water treatment for achieving higher efficiency. Ozone decomposition by H2O2 incorporation is initiated by *OH and superoxide formation.26 In case of treating surface waters using conventional ozonation and O3/H2O2 much difference was not observed in the transformation of ozone to HO*. But in case of groundwater, containing para-chlorobenzoic acid (pCBA) the oxidation of it by conventional ozone is 20% and was increased to 50 % by incorporating H2O2 along with O3.26 Compounds like geosmin and 2-methylisobrneol (MIB) produced by algae are also difficult to oxidize by ozone as they contain saturated ring system. However, they are well suited to be treated by O3/H2O2 having high oxidation rate constant [26].The Bromate formation is low in O3/H2O2 process than the conventional ozonation.51
O3/Catalyst
Homogeneous and heterogeneous catalyst addition in ozonation process also plays a key role in enhancing the oxidation reaction.57 Metal oxides like Fe2O3, Al2O3-Me, TiO2-Me, MnO2, Ru/CeO2 and metal ions like Fe2+, Fe3+, Mn2+, etc. have been used as catalysts.57 Successful removal of chemical oxygen demand, organochlorides, and total organic carbon had been achieved by combining iron and manganese.57,58 Combinations like O3/TiO2, Ru/CeO2/O3,33 Al2O3/O3 showed better efficacy in removing total organic matters [57]. Granular activated carbon is also considered as a catalyst for the destruction of bio-refractory compounds.57,60
UV bases AOP
The UV treatment is generally applied as a tertiary for killing the microbes and degradation of those aquatic organic compounds which can absorb UV light. On the absorption of UV light, the electrons in the pollutant excite from the ground state to the excited state, (equation. 1) from where the electrons are transferred to an oxygen molecule which converts both O2 and the pollutant molecule into a radical (equation. 2). The radical being a highly reactive species oxidizes other molecules to acquire a stable form.
P → P* ...............................(1)
P* + O2 → P+* + O2-* .....................(2)
UV light can also result in the homolytic cleavage of the chemical bond of the pollutants resulting in the formation of two radicals (equation. 3). UV light with a wavelength less than 190 nm can effectively break highly stable C-F bond whereas wavelength in the range 210-230 nm can break C-Cl bond. The radicals formed can then react with oxygen (equation. 4) or may take part in the further oxidation-reduction reactions with the other dissolve molecules.
R → X → R* + X* .................(3)
R* + O2 → RO2 ............................(4)
High energy UV lights with a wavelength smaller than 190 nm can photolyze water molecule to form *OH (equation. 5), that subsequently oxidize other organic substrates.61
H2O + hv → 2HO* ..........................(5)
Lamps like Mercury arc with different UV light emitting intensities are used for generation of the UV radiation. These are generally of three types: Low pressure, medium and high-pressure lamp. The former is monochromatic in nature and emit light of wavelength 253.7nm. Medium and high pressure lamps emit a wide range of wavelengths in the UV region and penetrate deeper because of their higher intensity and take less time for the completion of the treatment. The limitation with the application of them is that they are energy intensive.62 Lamps like Pulse radiation lamps and Excimer lamps were also utilized for generation of UV irradiation.63 The main component of UV treatment system includes lamps, ballast and a reactor. Mercury arc lamps are generally used as the UV lamps. Ballast is a support device, which mainly performs two functions: first, they provide appropriate voltage for the reliable starting of the lamp; secondly, they maintain a continuous current flow to the lamp, to prevent the lamps from short-circuiting. Finally, the Reactor, they are of two types: contact and non-contact reactor. The contact reactors are the one in which the UV lamps remain in submerged condition. The lamps are generally enclosed within quartz sleeves in order to prevent them from damages. Non-contact reactors are the one in which a transparent material is placed between the water sample and the lamps. The lamps can be positioned either in perpendicular or parallel to the wastewater flow direction.62,64 The major strength of this process is eco-friendly and shorter treatment time. It also does not require the use chemicals; therefore, no residual products are left after the treatment.62 This technique has been applied for the degradation of numerous organic compounds, including EDCs (Endocrine disrupting chemicals) and various industrial solvents. It is an effective method for degrading NDMA (N-nitrosodimethylamine) a well-known potent carcinogen and mostly found in wastewater in trace amounts.65-67 Sakai et al (2012) found 222 nm Kr- Excimer - UV lamps to be a better option for NDMA degradation in the place of low pressure (LP) and filtered medium pressure lamps (FMP).68 Sanches et. al. 2010 utilized low-pressure UV lamps to check the degradation of five pesticides viz: Alachlor, Atrazine, Diuron, Pentachlorophenol, Chlorphenvinphos and Isoproturon. More than 50% of all the pesticides were completely degraded by the conventional low-pressure mercury lamp except Isoproturon. The low quantum yield of Isoproturon, in spite of having high molar absorption coefficient, can be a reason behind its less degradation.69 Various categories of pollutants treated by UV photolysis are summarized in Table 2.
Table 2: Various categories of pollutants treated by UV photolysis
|
Category |
Specific Pollutant |
Ref. |
|
Trace Organic Chemical |
NDMA(N-nitrosodimethylamine) |
[68] |
|
Pharmaceuticals |
Diclofenac, Antipyrine, Chlorotetracycline, Norfloxacin, Caffeine, Dipyridamole, Diltiazem, Clofibric acid, Acetamiprid |
[70] |
|
sulfamethoxazole (SMX), oxytetracycline (OTC) and ciprofloxacin (CIP) |
[71] |
|
|
Ditrizoate |
[72] |
|
|
Pesticides |
Atrazine, Diuron, Alachlor, Pentachlorophenol, Chlorphenvinphos |
[69] |
|
Anabolic-androgenic steroid |
Boldenone |
[73] |
|
EDC |
Butylparaben |
[74] |
The limitations of the technique are the *OH formed by the action of UV, sometimes leads to the partial degradation of the organic contaminants, leaving behind the intermediates concentration in a higher level at the place of CO2 and H2O. The higher concentration of Total Suspended Solids (TSS) and particulate material (e.g. humic compounds and iron) lags the treatment. Further, it is energy intensive and expensive as compared to the other tertiary processes like chlorination.62
UV/O3 Process
Treatment by combining UV and O3 is a well-established advanced oxidation technology. This technique is more advantageous than the individual UV and O3 technologies since it combines the advantages of both. The ultraviolet light coming in contact with the ozone (O3) breaks it to form *OH, through the following reaction steps75:
O3 + H2O + hv → H2O2 + O2 .......................(6)
H2O2 + hv → 2OH* .......................(7)
2O3 + H2O2 → 2OH* ...........................(8)
The *OH formed, then either completely mineralizes the organic substances to form CO2 or H2O or form some easily degradable intermediate substances. Being an unstable and reactive molecule, O3 gas is generated in-situ in the experiment. Thus, the generated O3 is then sparged into the reactor in which UV lamps are installed.75,76 These UV lamps are enclosed within Quartz sleeves in the contact reactors, which ensure better transmission of UV. In the non-contact type UV lamp and wastewater are separated by a transparent separator. Schematics of a UV reactor configuration are shown in figure 5.
![Figure 5: Schematic representation of a UV reactor consisting of two UV Banks [77]](http://www.cwejournal.org/wp-content/uploads/2017/12/Vol12_No3_rev_Nir_Fig5-150x150.jpg) |
|
In most of the studies, it was found that the UV/O3 treatment was successful in TOC and COD reduction besides its pollutant degradation capacity.75,78-80 In a study performed by Hassan et. al. (2017), it was found that the color removal was higher for UV/O3 in comparison to individual treatment with O3.80 Tehrani et. al. (2010) studied the removal of Reactive Blue 19 dye through the UV/O3 and O3 treatment. In their study, it was found that the UV/O3 treatment was more effective in COD removal than the O3. It was also found that decolourization decreased as the initial concentration of dye was increased.78 The factors which can affect the treatment includes: pH, initial pollutant concentration, the turbidity of the solution, amount of O3 dose, amount of UV dose, types of UV lamps used for the process, reaction time, scavengers present in the water.80-82 Table 4 enlists some selected pollutants removed effectively by UV/O3 treatment
Table 3: Selected Pollutants Removed By UV/O3 Treatment
|
Category |
Specific pollutant |
Reference |
|
Dyes |
RB-19 |
[79] |
|
DB-86 |
[80] |
|
|
MV-40 |
[81] |
|
|
Direct Yellow 50 |
[82] |
|
|
Phenols |
4-Chlorophenol |
[83] |
|
Pharmaceuticals |
Ketoprofen |
[84] |
|
Caffeine |
[85] |
|
|
Pesticides |
Linuron |
[86] |
|
Other organic chemicals |
Nitrosopyrollidine |
[87] |
|
Bisphenol A |
[88] |
Fenton Processes
The Fenton is one of the prominent AOP and the chemistry of reactions related to this includes reactions of peroxides (H2O2) with Fe2+ to generate reactive oxygen species that can degrade the organic as well as inorganic matter in aqueous phase. Fenton chemistry dates back to 1894 when the activation of H2O2 by ferrous salts was reported for oxidizing tartaric acid by Henry J.Fenton.89 In 1934 it was proposed by Haber and Weiss90 that there is a formation of *OH due to the Fenton reaction. This *OH has an oxidation potential of 2.73V. Hence it is one of the most active and powerful oxidants, which can be used in the degradation of most organic compounds including emerging.91
Homogeneous Fenton reactions
The Fenton process is an easy and economical method to generate highly reactive oxygen species for degrading contaminants. H2O2 is safe and easy to handle, comparatively cheap and easily decomposes into water and oxygen. Similarly, iron is also cheaper and safe to use. This mechanism of decomposition of H2O2 was later revised by Barb et al.92-94. to introduce the chain reactions of the Fenton process. The proposed mechanism which involves the breaking down of H2O2 to produce *OH /free radical is a sequence of seven reactions in an acidic medium under dark conditions.95 The reactions are as follows:
Fe2+ + H2O2 → Fe3+ + OH− + *OH ..........................(9)
Fe3+ + H2O2 → Fe2+ + HO·2 + H+ ...........................................(10)
*OH + H2O2 → HO·2 + H2O .............................(11)
*OH + Fe2+ → Fe3+ + OH− ................................(12)
Fe3+ + HO·2 → Fe2+ + O2H+ .................................(13)
Fe2+ + HO·2 + H+ → Fe3+ + H2O2 ..............................(14)
HO·2 + HO·2 → H2O2 + O2 ...................................(15)
*OH, which is the required oxidant for degradation of the contaminants is produced by reaction 9. Reaction 10, therefore, becomes the rate-limiting reaction because it is slower than proceeding by several orders of magnitude. The organic compounds (RH / R) can be oxidized by any one or combinations of the following: (1) *OH, (2) abstraction of hydrogen (R*), (3) addition of hydroxyl (*ROH).96
RH + *OH → H2O + R* → Further oxidation ..................(16)
R + *OH → *ROH → Further oxidation ..............................(17)
*OH, can be scavenged by either Fe2+ or H2O2 as shown in equation 10 and 11. Hence optimization of Fe2+ / H2O2 is to be carried for reducing the scavenging of ·OH. Whereas, the produced Fe3+ precipitates to form ferric oxy-hydroxides as the pH increases from its optimum generating an undesirable sludge giving problems in practical applications.97,98 Additionally, the drawbacks of application of Fenton process for large-scale wastewater treatment include pH dependency (effective only in the range of pH 2 to 5), generation of iron-based sludge, the necessity of its expensive post-treatment and finally neutralizing the treated water before disposal.99 Therefore, further research is required on modified Fenton processes such as photo Fenton which can increase the reaction rate by light irradiation. Sometimes the use of chelating agents provides optimum pH for Fenton reactions. Chelating ligands compete favorably with hydroxide ion; hence increase the pH range over which compounds are soluble. The reaction kinetics is as similar to Fenton oxidation at the optimum pH. Various chelators include Fe3+-CIP chelate, deferioxamine, cyclodextrin, nitrilotriacetic acid (NTA) and ethylenediaminetetraacetic acid (EDTA)100-103 were adopted. However, additional research is required to identify chelating agents that can increase the rate of oxidation, stability, and eco-friendliness.
Photo – Fenton Process
Irradiating Fenton reaction solutions with ultraviolet and visible light increases its reaction rate and the efficiency towards waste degradation.99 The increase in efficiency is correlated to the photochemistry of Fe3+ complexes [Fe3+(OH)-] + and [Fe3+(RCO2)-]2+ to dissociate into Fe2+. The photochemistry of Fe3+ gives an advantage to Fenton processes because the reduced Fe2+ reacts with H2O2 forming *OH as per reaction 9.104 The photo-Fenton reaction reaches its optimum around pH 2.8 and as the pH increases above this the Fe3+ precipitates as oxyhydroxides and as pH decreases below the optimum, the concentration of Fe(OH)2+ will decline.105 The Photolysis of hydrogen peroxide takes place under UV light irradiation as shown (equation 18):
H2O2 + hν → 2HO* ................................(18)
Although the quantum yield by photolysis of hydrogen peroxide shown in reaction 10 is good, due to the weak absorption of light irradiation by H2O2, its activity in photo Fenton reactions is not significant. Similar to Fenton, photo-Fenton has the drawbacks of high cost for sludge processing and the requirement of narrow pH range, limits its application in waste/wastewater treatment.97,106
Heterogenous Photocatalysts
Heterogeneous photocatalysis is different compared to other treatment methods involving oxidation and reduction simultaneously, with the use of light irradiation and a light absorbing photocatalyst. Various compounds can utilize light irradiation to catalytically undergo photolysis to produce redox reactions. These compounds usually have a band structure with empty conduction and a filled valence band.89 Once light irradiation is incident on a semiconductor and if the energy of photons exceeds the energy gap of the semiconductor, electrons are excited from valence band to the conduction band leaving behind the holes.90,91 The activated electrons in the valence band and holes in the conduction reacts with water generating *OH, superoxide (*O2-) and peroxide radicals (*OOH). These radicals further degrade various pollutants to obtain products such as CO2 and H2O.92-94 The mechanism of photocatalysis and degradation of contaminants is illustrated in figure 6.
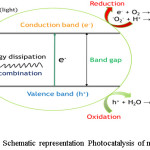 |
|
The heterogonous photocatalyst materials consist of TiO2, ZnO, Fe2O3, WO3, SnO2, ZrO2, CdS ZnS, etc. Among them, TiO2 is the most extensively used heterogonous photocatalyst due to its easy availability, cheapness and high chemical stability. The other classification of photocatalyst also experimented for its applicability. Owing to the limitation of utilizing the visible light the conventional photocatalyst materials were hybridized with suitable cocatalyst. Table 4 shows the various hybridized heterogeneous photocatalysts utilized for waste degradation.
Table 4: Various photocatalysts studied for degrading different pollutants
| Sl. No. | Photocatalyst | Pollutant | Removal efficiency | Ref. |
| 1. | Graphene oxide/WS2/Mg doped ZnO nanocomposite. | Rhodamine B (RhB) | 90% in 5 min | [98] |
| 2. | Pd-TiO2 photocatalyst | Amoxicillin (AMX) | 97.5% in 5h | [107] |
| 3. | Hexagonal WS2 platelets | Rhodamine B | 98% in 105min | [99] |
| 4. | Ag+, Fe3+ and Zn2+ intercalated cadmium(II) metal organic frameworks | 2-cholrophenol (2CP) | 93% in 5h | [104] |
| 5. |
Reduced graphene oxide and Ag wrapped TiO2 |
Bisphenol A (BPA) |
69.1% in 5h |
[105] |
| 6. |
Graphene bridged Ag3PO4/Ag/BiVO4 photocatalyst |
Tetracycline |
94.96% removal in 60min |
[106] |
|
7. |
Magnetic biochar supported g-C3N4/FeVO4
|
methyl paraben (MeP) and 2-cholrophenol (2-CP) |
98.4% MeP removal and 90.7% 2-CP removal |
[100] |
|
8. |
In-based MOF/graphene oxide |
Amoxicillin (AMX) |
93% AMX removal, 80% TOC removal |
[101] |
|
9. |
Magnetic RGO/ZnO/ZnFe2O4 composite |
Methylene Blue (MB) |
98.64% in 60min |
[102] |
|
11. |
Fe3O4 nanoparticles and oxalate complex |
Phenol |
97.61% in 3h |
[103] |
|
12. |
Copper zinc tin sulfide (CZTS) nanoparticles | Methylene blue (MB) dye | 50% in 45min | [108] |
|
13. |
Reticulated ZnO | Acid Red 88 dye | 79% in 180min | [109] |
|
14. |
α-Fe2O3 nanosheets |
Bisphenol S | 91% in 120min | [110] |
|
15. |
Ag/TiO2 photocatalyst |
Amoxicillin (AMX), 2,4-dichlorophenol (DCP) |
63.48% AMX & 60.23% 2,4-DCP in 5h |
[111] |
Fenton reactions using Heterogeneous catalysts
In conventional Fenton process removal of dissolved iron from the treated water is one of the biggest challenges which prompted the use of heterogeneous catalysts along with Fenton process. The investigation has been carried out on the use of supported iron catalysts to decrease sludge formation and to increase the pH range over which the Fenton reaction is effective. In Heterogeneous catalysts supported Fenton process reactant molecules get adsorbed on the active sites present on the surface of the catalyst. The products get desorbed after the reaction takes place.112 The use of goethite (-FeOOH), hematite (-Fe2O3), FeS2/SiO2, Ilmenite (FeTiO3) and Titanomagnetite (Fe3TiO4) as heterogeneous Fenton catalysts has been studied by various researchers.113-116 The advantage of an ideal heterogeneous catalyst is its ability to separate from water and function in wide range of pH. Various pollutants degraded using Fenton/ Heterogeneous catalysts are tabulated in table 5.
Table 5: Pollutants degraded using Fenton/ Heterogeneous catalysts
|
Sl. No |
Fenton/ Heterogeneous catalysts |
Pollutant |
Removal Efficiency |
Ref. |
|
1. |
Photo-Fenton/ magnetite and EDDS |
Bisphenol A (BPA) |
BPA = 70% in 11h |
[117] |
|
2. |
Bioelectro-Fenton /carbon felt cathode /boron-doped diamond anode |
Pharmaceutical wastewater |
COD = 60%, 5-fluorouracil = 88%, caffeine = 43% in 3days |
[118] |
|
3. |
Fenton/ FeS2/SiO2 |
Ciproflaxacin |
Ciproflaxacin = 99% in 60 min |
[113] |
|
4. |
Fenton/ ferric sludge |
Landfill leachate |
BOD7 = 99%, COD = 86% |
[119] |
|
5. |
Photo-Fenton |
Micropollutants from municipal wastewater |
Micropollutants = 40% |
[120] |
|
6. |
Fenton with graphene modified iron sludge as catalyst |
Rhodamine B (RhB), acid red G (ARG), metronidazole |
RhB = 99%, ARG = 98.5%, metronidazole = 91.8% |
[121] |
|
7. |
Photo Fenton oxidation with zeolite as catalyst |
Paracetamol |
Paracetamol removal = ~99%, TOC removal = 60% in 5h |
[122] |
Other AOPs
The above discussed processes are the most prominence among the AOPs with practical applicability. Apart there are few like wet air oxidation (WAO), supercritical wet oxidation process (SWOP) process and electron beam radiation. Off this WAO utilizes the molecular oxygen or air as oxidizer in high pressure and temperature environment. This extreme condition allows the generation of free radicals that decomposes the waste.123 Thus temperature and pressure are the controlling factor for WAO process. Most of the organic acids excluding acetic and propionic acid124 are converted to CO2 at high temperature. Figure 7 depicts the simple functionalities of wet oxidation.
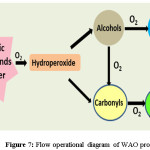 |
|
Huge operational expenditure and investment is required to maintain a WAO system as it handles an extreme reaction environment. Figure 8 illustrates Reactor design adopted for WAO system.
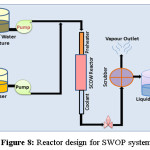 |
|
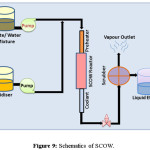
SWOP employs supercritical condition refers to the extreme temperature and pressurized condition, where water changes its polarity and became a non-polar solvent to get miscible with the organic part. In supercritical condition, water can be mixed with the oxygen creating a homogeneous mixture which is perfect medium for organic degradation. When organic compounds and oxygen are dissolved in water above the critical point they are immediately brought into intimate molecular contact in a single homogeneous phase. With no interface transport limitations at sufficiently high temperatures, the kinetics is fast and the oxidation reaction proceeds rapidly to completion. This supercritical water oxidation (SCOW) is also referred as hydrothermal oxidation (HTO). This process uses hydrogen peroxide in a homogeneous mixture to oxidize the toxic effluents above the critical point of water. If the organic material is in solid phase it requires the heterogeneous catalytic SCOW. It is most efficient method in case of environmental remediation as complete removal of oxidisable material can be achieved by this process. Till date inorganic substances like ammonia or cyanides can also be converted to CO2, H2O, and N2. SCOW consists of four steps, starting from pressurizing the reagent, reaction, salt separation and depressurization. Wastewater and oxidant are mixed and fed in the SCOW reactor. The oxidants are normally heated before reaction and this reaction condition allows the exothermic reaction between the waste and oxidant. The heat energy obtained in the oxidation reaction helps to activate the reagent optimally to oxidizable condition. After the treatment the salt precipitation is visible inside the reactor, because of reduced salt solubility. Though the process requires high energy, this demand can be recovered by utilization of this hot stream for preheating or energy production. A number of works that study in depth the organic reactions in SCW, focusing on the influence of the properties of water and in the kinetics modelling, has been developed over the last few years. The table 6 consists of the list of some organizations that have incorporated SCOW plant for water treatment. Figure 9 shows the schematics of a SWOP process for water/wastewater treatment while the table 6 lists the organization that incorporated the same.
 |
|
Table 6: List of some organizations that have incorporated SCOW plant for water treatment
|
Compound |
Treated Effluent |
Removal efficiency |
Ref. |
|
ETH Zurich (Switzerland) |
Methanol |
TOC rem. 99.9% |
[125] |
|
ITC-CPV Karlsruhe (Germany) |
Ethanol, Na2SO4, Paper mill waste effluents |
TOC rem. 99.9% |
[126] |
|
Sandia’s EER (USA) |
Isopropyl alcohol Military smokes and dye formulations |
No Na2SO4 deposits in the transpiring wall |
[127] |
|
Pine Bluff Arsenal (USA) |
Isopropyl alcohol Sugar and Na2SO4 Solutions Military smokes and dye formulations |
TOC rem. 99.9% |
[128] |
|
U.S. Navy/Army’s Dugway Proving Ground Facility (Utah, USA) |
Naval wastes (Cl- and Fl- containing) (71 h) Chemical weapons (231 h) |
TOC rem.> 99.9% (Trials for Blue Grass chemical weapons destruction) |
[129] |
|
CEA (France) |
Methanol |
TOC rem. 99.9% |
[130] |
Conclusion
The review explicitly discussed on the robustness of various AOPs that has been extensively used for water management. Their treatment efficiency depended on various process parameters that were clearly reviewed. However, limitations such as high operational costs, high energy consumption and lower generation of the reactive species initiated the need for process intensifications. The strength of the intensified system clarified their role on treating various pollutants. Though various AOPs for water treatment are discussed, the Heterogeneous Photocatalysis is most widely preferred over the rest of the processes for real-time applications. Over all the review presented the clear scenario of the AOPs for water and wastewater treatment with a benefit to the community.
Acknowledgment
The corresponding author is grateful to Science and Engineering Research Board (SERB), Department of science and Technology for the financial support received under Early Career Research Award with grant code ECR/2016/001400.
References
- Akar, S. T., & Uysal, R. Untreated clay with high adsorption capacity for effective removal of CI Acid Red 88 from aqueous solutions: Batch and dynamic flow mode studies. Chemical Engineering Journal. 2010;162(2):591-598.
- Biń, A. K., & Sobera-Madej, S. Comparison of the advanced oxidation processes (UV, UV/H2O2 and O3) for the removal of antibiotic substances during wastewater treatment. Ozone: Science & Engineering. 2012;34(2):136-139.
- Abdel-Raouf, N., Al-Homaidan, A. A., & Ibraheem, I. B. M. Microalgae and wastewater treatment. Saudi Journal of Biological Sciences. 2012;19(3): 257-275.
- Tsydenova, O., Batoev, V., & Batoeva, A. Solar-enhanced advanced oxidation processes for water treatment: Simultaneous removal of pathogens and chemical pollutants. International journal of environmental research and public health. 2015;12(8):9542-9561.
- Geyer, H. J., Rimkus, G. G., Scheunert, I., Kaune, A., Schramm, K. W., Kettrup, A., & Mackay, D. Bioaccumulation and occurrence of endocrine-disrupting chemicals (EDCs), persistent organic pollutants (POPs), and other organic compounds in fish and other organisms including humans. In Bioaccumulation–New Aspects and Developments(pp. 1-166). Springer Berlin Heidelberg. 2000.
- Liu, Y., He, X., Fu, Y., & Dionysiou, D. D. Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes. Chemical Engineering Journal. 2016;284:1317-1327.
- Ayoub, K., van Hullebusch, E. D., Cassir, M., & Bermond, A. Application of advanced oxidation processes for TNT removal: a review. Journal of hazardous materials. 2010;178(1):10-28.
- Bebelis, S., Bouzek, K., Cornell, A., Ferreira, M. G. S., Kelsall, G. H., Lapicque, F., & Walsh, F. C. Highlights during the development of electrochemical engineering. Chemical Engineering Research and Design. 2013;91(10):1998-2020.
- Bethi, B., Sonawane, S. H., Bhanvase, B. A., & Gumfekar, S. P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chemical Engineering and Processing: Process Intensification. 2016;109:178-189.
- Fernándezâ€Castro, P., Vallejo, M., San Román, M., & Ortiz, I. Insight on the fundamentals of advanced oxidation processes. Role and review of the determination methods of reactive oxygen species. Journal of Chemical Technology and Biotechnology. 2015;90(5):796-820.
- Glaze, W. H. Drinking-water treatment with ozone. Environmental science & technology. 1987;21(3):224-230.
- Glaze, W. H., Kang, J. W., & Chapin, D. H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. 1987.
- Joseph, C. G., Puma, G. L., Bono, A., & Krishnaiah, D. Sonophotocatalysis in advanced oxidation process: a short review. Ultrasonics Sonochemistry. 2009;16(5):583-589.
- Chowdhury, P., & Viraraghavan., T. Sonochemical degradation of chlorinated organic compounds, phenolic compounds and organic dyes – A review. Science of the Total Environment. 2009;407:2474–2492.
- Sathishkumar, P., Mangalaraja, R. V., & Anandan, S. Review on the recent improvements in sonochemical and combined sonochemical oxidation processes–A powerful tool for destruction of environmental contaminants. Renewable and Sustainable Energy Reviews. 2016;55:426-454.
- Patil, M. N., & Pandit, A. B. Cavitation—a novel technique for making stable nanosuspensions. Ultrasonics Sonochemistry. 2007;14:519–530.
- Doosti, M. R., Kargar, R., & Sayadi, M. H. Water treatment using ultrasonic assistance: a review. Proceedings of the International Academy of Ecology and Environmental Sciences. 2012;2(2):96–110.
- Entezari, M. H., Mostafai, M., & Sarafraz-yazdi, A. A combination of ultrasound and a bio-catalyst: removal of 2-chlorophenol from aqueous solution. Ultrasonics Sonochemistry. 2006;13:37–41.
- Mahvi, A. H. Application of ultrasonic technology for water and wastewater treatment. Iranian Journal of Public Health. 38(2):1–17.
- Tuziuti, K. Yasui, Y. Iida, H. Taoda, S. Koda, Ultrasonics. 2009;42(1–9),(2004)597–601.
- Selli, E. Synergistic effects of sonolysis combined with photocatalysis in the degradation of an azo dye. physical chemistry chemical physics. 2002;4(24), 6123-6128.
- Vecitis, C.D., Lesko, T., Colussi, A.J., Hoffmann, M.R., Sonolytic decomposition of aqueous bioxalate in the presence of ozone. J. Phys. Chem. A 2010;114:4968e4980.
- Wang, X., Wang, J., Guo, P., Guo, W., & Wang. C., Degradation of rhodamine B in aqueous solution by using swirling jet-induced cavitation combined with H2O2. Journal of Hazardous Materials. 2009;169:486–491.
- Destaillats, H., Colussi, A.J., Joseph, J.M., Hoffmann, M.R., Synergistic effects of sonolysis combined with ozonolysis for the oxidation of azobenzene and methyl orange. J. Phys. Chem. A. 2000;104:8930e8935.
- Glaze, W. H., Kang, J. W., and Chapin, D. H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. 1987.
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water research. 2003;37(7):1443-1467.
- Konsowa, A. H. Decolorization of wastewater containing direct dye by ozonation in a batch bubble column reactor. Desalination. 2003;158(1):233-240.
- Hoigné, J. Chemistry of aqueous ozone and transformation of pollutants by ozonation and advanced oxidation processes. In Quality and treatment of drinking water II: 83-141. Springer Berlin Heidelberg. 1998.
- Bourgin, M., Borowska, E., Helbing, J., Hollender, J., Kaiser, H. P., Kienle, C., and von Gunten, U. Effect of operational and water quality parameters on conventional ozonation and the advanced oxidation process O 3/H 2 O 2: Kinetics of micropollutant abatement, transformation product and bromate formation in a surface water. Water Research. 2017;122:234-245.
- Gottschalk, C., Libra, J. A., and Saupe, A. Ozonation of water and waste water: A practical guide to understanding ozone and its applications. John Wiley and Sons. 2009.
- Camel, V., and Bermond, A. The use of ozone and associated oxidation processes in drinking water treatment. Water research, 32(11): 3208-3222. (1998)
- Badia-Fabregat, M., Oller, I., and Malato, S. Overview on Pilot-Scale Treatments and New and Innovative Technologies for Hospital Effluent. (2017).
- Staehelin, J., and Hoigne, J. Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions. Environmental Science and Technology, 19(12): 1206-1213. (1985)
- Sehested, K., Holcman, J., and Hart, E. J. Rate constants and products of the reactions of e-aq, dioxide (1-) (O2-) and proton with ozone in aqueous solutions. The Journal of Physical Chemistry, 87(11): 1951-1954. (1983)
- Stettler, R., Courbat, R., von Gunten, U., Kaiser, H. P., Walther, J. L., Gaille, P., and Revelly, P. Utilisation de l'ozone pour le traitement des eaux potables en suisse. GWA, 78(11): 876-890. (1998)
- Exner, O. Correlation analysis of chemical data. Springer. (1988)
- Stumm, W., and Morgan, J. J. Aquatic Chemistry, 3rd. John Wiley and Sons, New York. (1996)
- Hitzfeld, B. C., Höger, S. J., and Dietrich, D. R. Cyanobacterial toxins: removal during drinking water treatment, and human risk assessment. Environmental health perspectives, 108(Suppl 1), 113.Hoigné, J., and Bader, H. (1983). Rate constants of reactions of ozone with organic and inorganic compounds in water—II: dissociating organic compounds. Water research, 17(2): 185-194. (2000)
- Haag, W. R., and Yao, C. D. Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environmental Science and Technology, 26(5): 1005-1013. (1992)
- Dowideit, P., and von Sonntag, C. Reaction of ozone with ethene and its methyl-and chlorine-substituted derivatives in aqueous solution. Environmental Science and Technology, 32(8): 1112-1119. (1998)
- Muñoz, F., and von Sonntag, C. The reactions of ozone with tertiary amines including the complexing agents nitrilotriacetic acid (NTA) and ethylenediaminetetraacetic acid (EDTA) in aqueous solution. Journal of the Chemical Society, Perkin Transactions 2, (10): 2029-2033. (2000)
- Huber, M. M., Canonica, S., Park, G. Y., and Von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environmental science and technology, 37(5): 1016-1024. (2003)
- Pryor, W. A., Giamalva, D. H., and Church, D. F. Kinetics of ozonation. 2. Amino acids and model compounds in water and comparisons to rates in nonpolar solvents. Journal of the American Chemical Society, 106(23): 7094-7100. (1984)
- Muñoz, F., Mvula, E., Braslavsky, S. E., and von Sonntag, C. Singlet dioxygen formation in ozone reactions in aqueous solution. Journal of the Chemical Society, Perkin Transactions 2, (7): 1109-1116. (2001)
- Rennecker, J. L., Mariñas, B. J., Owens, J. H., and Rice, E. W. Inactivation of Cryptosporidium parvum oocysts with ozone. Water Research, 33(11): 2481-2488. (1999)
- Reungoat, J., Escher, B. I., Macova, M., and Keller, J. Biofiltration of wastewater treatment plant effluent: effective removal of pharmaceuticals and personal care products and reduction of toxicity. Water research, 45(9): 2751-2762. (2011)
- Andreozzi, R., Caprio, V., Insola, A., and Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catalysis today, 53(1): 51-59. (1999)
- Duguet, J. P., Brodard, E., Dussert, B., and Mallevialle, J. Improvement in the effectiveness of ozonation of drinking water through the use of hydrogen peroxide. (1985)
- Pinkernell, U., and von Gunten, U. Bromate minimization during ozonation: mechanistic considerations. Environmental Science and Technology, 35(12): 2525-2531. (2001)
- Glaze, W. H., Kang, J. W., and Chapin, D. H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. (1987)
- Hango, R. A., Doane, F., and Bollyky, L. J. Wastewater treatment for reuse in integrated circuit manufacturing. Wasser Berlin, 81(5): 304. (1981)
- Bollyky, L. J. Pilot Plant Studies for THM, Taste and Odor Control Using Ozone and Ozone-Hydrogen Peroxide. In the Role of Ozone in Water and Wastewater Treatment. Proceedings of the Second International Conference. DW Smith and GR Finch, Eds. (Kitchener, Ontario, Canada: TekTran International, Inc., 1987): 211. (1987)
- Brunet, R., Bourbigot, M. M., and Dore, M. Oxidation of organic compounds through the combination ozone–hydrogen peroxide. (1984)
- Duguet, J. P., Brodard, E., Dussert, B., and Mallevialle, J. Improvement in the effectiveness of ozonation of drinking water through the use of hydrogen peroxide. (1985)
- Munter, R. Advanced oxidation processes–current status and prospects. Estonian Acad. Sci. Chem, 50(2): 59-80. (2001)
- Cortes, S., Sarasa, J., Ormad, P., Gracia, R., and Ovelleiro, J. L. Comparative Efficiency of the Systems O3/High pH And O3/catalyst for the Oxidation of Chlorobenzenes in Water. Ozone: science and engineering, 22(4): 415-426. (2000)
- Karpel Vel Leitner, N., Delanoe, F., Acedo, B., Papillault, F., and Legube, B. Catalytic ozonation of succinic acid in aqueous solution: A kinetic approach. In Int. Reg. Conf. Ozonation and AOPs in Water Treatm., September 23–25. (1998)
- Pinker, B., and Henderson, W. D. The effect of ozonation on the performance of GAC. In Reg. Conf. Ozone, UV-light, AOPs Water Treatm., September 24–26, 1996, Amsterdam, Netherlands: 307-318. (1996)
- Zwiener, C. F. F. H., and Frimmel, F. H. Oxidative treatment of pharmaceuticals in water. Water Research, 34(6): 1881-1885. (2000)
- Valdés, H., Schrickel, K., Bormann, H., and Sievers, M. Ozonation of pentylacetate contaminated waters from textile care industry. Obras y Proyectos, (13). (2013)
- Legrini, O., Oliveros, E., and Braun, A. M. Photochemical processes for water treatment. Chemical reviews, 93(2): 671-698. (1993).
- Matasci, R., Weston, R., Lau, P., Cruver, J., Marek, S., & Tomowich, D. (1999). Wastewater Technology Fact Sheet: Ultraviolet Disinfection, United States Environmental Protection Agency, Office of Water, Washington, DC. EPA 832-F-99-064.
- Giller, H.F.J.I, A Review of Lamp Types, Proceedings Disinfection 2000. Water Environment Federation, (2000).
- Wastewater Ultraviolet Disinfection. Retrieved from iwa-network.org.
- Lee, C.H., Choi, W.Y., Kim, Y.G., Yoon, J., UV photolytic mechanism of Nnitrosodimethylamine in water: dual pathways to methylamine versus dimethylamine. Environ. Sci. Technol. 39, 2101–2106. (2005a)
- Lee, C.H., Choi, W.Y., and Yoon, J. UV photolytic mechanism of N-nitrosodimethylamine in water: Roles of dissolved oxygen and solution pH. Environ. Sci. Technol. 39, 9702–9709. (2005)
- Sharpless, C.M., and Linden, K.G. Experimental and model comparisons of low and medium-pressure Hg lamps for the direct and H2O2 assisted UV photodegradation of N-Nitrosodimethylamine in simulated drinking water. Environ. Sci. Technol. 37: 1933–1940. (2003).
- Sakai, H., Takamatsu, T., Kosaka, K., Kamiko, N., and Takizawa, S. Effects of wavelength and water quality on photodegradation of N-Nitrosodimethylamine (NDMA). Chemosphere, 89(6):702-707. (2012).
- Sanches, S., Crespo, M. T. B., & Pereira, V. J. Drinking water treatment of priority pesticides using low pressure UV photolysis and advanced oxidation processes. Water Research, 44(6), 1809-1818. (2010)
- Kim, I., Yamashita, N., and Tanaka, H. Performance of UV and UV/H 2 O 2 processes for the removal of pharmaceuticals detected in secondary effluent of a sewage treatment plant in Japan. Journal of Hazardous Materials, 166(2): 1134 -1140. (2009).
- Avisar, D., Lester, Y., and Mamane, H. pH induced polychromatic UV treatment for the removal of a mixture of SMX, OTC and CIP from water. Journal of hazardous materials, 175(1): 1068-1074. (2010).
- Real, F. J., Benitez, F. J., Acero, J. L., Sagasti, J. J., and Casas, F. Kinetics of the chemical oxidation of the pharmaceuticals primidone, ketoprofen, and diatrizoate in ultrapure and natural waters. Industrial & Engineering Chemistry Research, 48(7):3380-3388. (2009).
- Błędzka, D., Gmurek, M., Gryglik, M., Olak, M., Miller, J. S., and Ledakowicz, S. Photodegradation and advanced oxidation of endocrine disruptors in aqueous solutions. Catalysis Today, 151(1): 125-130. (2010).
- Błędzka, D., Gmurek, M., Olak-Kucharczyk, M., Miller, J. S., and Ledakowicz, S. Photodegradation of n-butylparaben in natural water from Sulejow Reservoir. Chem. Eng. S 2012a, 19: 517-25. (2011).
- Hassaan, M. A., and El Nemr, A. Advanced Oxidation Processes for Textile Wastewater Treatment. International Journal of Photochemistry and Photobiology, 2(3):85-93. (2017).
- Rao, Y. F., and Chu, W. A new approach to quantify the degradation kinetics of linuron with UV, ozonation and UV/O 3Chemosphere, 74(11):1444-1449. (2009).
- Munter, R. Advanced oxidation processes–current status and prospects. Estonian Acad. Sci. Chem, 50(2): 59-80. (2001).
- Tehrani-Bagha, A. R., and Amini, F. L. Decolorization of a reactive dye by UV-enhanced ozonation. Progress in Color, colorants and coatings, 3:1-8. (2010).
- Hassaan, M. A., El Nemr, A., and Madkour, F. F. Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. The Egyptian Journal of Aquatic Research, 43(1): 11-19. (2017).
- Hassaan, M. A., El Nemr, A., and Madkour, F. F. Advanced oxidation processes of Mordant Violet 40 dye in freshwater and seawater. The Egyptian Journal of Aquatic Research, 43(1): 1-9. (2017).
- Hassaan, M. A., El Nemr, A., and Madkour, F. F. Application of Ozonation and UV assisted Ozonation for Decolorization of Direct Yellow 50 in Sea water. The Pharmaceutical and Chemical Journal, ISSN: 2349-7092, 3(3):131-138. (2016).
- Gong, J., Liu, Y., and Sun, X. O3 and UV/O3 oxidation of organic constituents of biotreated municipal wastewater. Water research, 42(4): 1238-1244. (2008).
- Lucas, M. S., Peres, J. A., and Puma, G. L. Treatment of winery wastewater by ozone-based advanced oxidation processes (O 3, O 3/UV and O 3/UV/H 2 O 2) in a pilot-scale bubble column reactor and process economics. Separation and Purification Technology, 72(3): 235-241. (2010).
- Souza, F. S., and Féris, L. A. Degradation of caffeine by advanced oxidative processes: O3 and O3/UV. Ozone: Science & Engineering, 37(4):379-384. (2015).
- Rao, Y. F., and Chu, W. A new approach to quantify the degradation kinetics of linuron with UV, ozonation and UV/O 3Chemosphere, 74(11):1444-1449. (2009).
- Chen, Z., Fang, J., Fan, C., and Shang, C. Oxidative degradation of N-Nitrosopyrrolidine by the ozone/UV process: Kinetics and pathways. Chemosphere, 150:731-739. (2016).
- Dudziak, M., and Burdzik, E. Oxidation of bisphenol A from simulated and real urban wastewater effluents by UV, O3 and UV/O3. Desalination and Water Treatment, 57(3):1075-1083. (2016).
- Barik, A. J., and Gogate, P. R. Degradation of 4-chloro-2-aminophenol using combined strategies based on ultrasound, photolysis and ozone. Ultrasonics sonochemistry, 28:90-99. (2016).
- Fenton, H. J. H. Oxidation of tartaric acid in presence of iron. Journal of the Chemical Society, Transactions, 65: 899-910 (1894)
- Haber, F., & Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proceedings of the Royal Society of London A: Mathematical, Physical and Engineering Sciences,147(861): 332-351 (1934)
- Munter, R. Advanced oxidation processes–current status and prospects. Estonian Acad. Sci. Chem, 50(2): 59-80 (2001)
- Barb, W. G., Baxendale, J. H., George, P., & Hargrave, K. R. Reactions of ferrous and ferric ions with hydrogen peroxide. Nature, 163(4148): 692-694 (1949)
- Barb, W. G., Baxendale, J. H., George, P., & Hargrave, K. R. Reactions of ferrous and ferric ions with hydrogen peroxide. Part I.—The ferrous ion reaction. Transactions of the Faraday Society, 47: 462-500 (1951a)
- Barb, W. G., Baxendale, J. H., George, P., & Hargrave, K. R. Reactions of ferrous and ferric ions with hydrogen peroxide. Part II. —The ferric ion reaction. Transactions of the Faraday Society, 47: 591-616 (1951b)
- Walling, C. Fenton's reagent revisited. Accounts of chemical research, 8(4): 125-131 (1975)
- Walling, C., & Kato, S. Oxidation of alcohols by Fenton's reagent. Effect of copper ion. Journal of the American Chemical Society, 93(17): 4275-4281 (1971)
- Pignatello, J. J., Oliveros, E., & MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Critical reviews in environmental science and technology, 36(1): 1-84 (2006)
- Kavitha, V., & Palanivelu, K. The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere, 55(9): 1235-1243 (2004)
- Hansson, H., Kaczala, F., Marques, M., & Hogland, W. Photo-Fenton and Fenton oxidation of recalcitrant industrial wastewater using nanoscale zero-valent iron. International Journal of Photoenergy, 2012(2012): 1-11. (2012).
- Chen, Y., Wang, A., Zhang, Y., Bao, R., Tian, X., & Li, J. Electro-Fenton degradation of antibiotic ciprofloxacin (CIP): Formation of Fe3+-CIP chelate and its effect on catalytic behavior of Fe2+/Fe3+ and CIP mineralization. Electrochimica Acta, 256: 185-195. (2017).
- Wang, N., Jia, D., Jin, Y., Sun, S. P., & Ke, Q. Enhanced Fenton-like degradation of TCE in sand suspensions with magnetite by NTA/EDTA at circumneutral pH. Environmental Science and Pollution Research, 24(21): 17598-17605. (2017).
- Bertrand, R. L. Iron accumulation, glutathione depletion, and lipid peroxidation must occur simultaneously during ferroptosis and are mutually amplifying events. Medical Hypotheses, 101: 69-74. (2017).
- Gogoi, A., Navgire, M., Sarma, K. C., & Gogoi, P. Novel highly stable β-cyclodextrin fullerene mixed valent Fe-metal framework for quick Fenton degradation of alizarin. RSC Advances, 7(64): 40371-40382. (2017).
- Giroto, J. A., Teixeira, A. C. S. C., Nascimento, C. A. O., & Guardani, R. Photo-Fenton removal of water-soluble polymers. Chemical Engineering and Processing: Process Intensification, 47(12): 2361-2369. (2008).
- Khataee, A., Salahpour, F., Fathinia, M., Seyyedi, B., & Vahid, B. Iron rich laterite soil with mesoporous structure for heterogeneous Fenton-like degradation of an azo dye under visible light. Journal of Industrial and Engineering Chemistry, 26: 129-135. (2015).
- De la Cruz, N., Giménez, J., Esplugas, S., Grandjean, D., De Alencastro, L. F., & Pulgarin, C. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Research, 46(6): 1947-1957. (2012).
- Burbano, A. A., Dionysiou, D. D., Suidan, M. T., & Richardson, T. L. Oxidation kinetics and effect of pH on the degradation of MTBE with Fenton reagent. Water Research, 39(1): 107-118. (2005).
- KoÄanová, V., & Dušek, L. Electrochemical dissolution of steel as a typical catalyst for electro-Fenton oxidation. Monatshefte für Chemie-Chemical Monthly, 147(5), 935-941. (2016).
- Abdelaziz, A. A. M., Nosier, S. A., & Hussien, M. Treatment of industrial wastewater containing phenol using the electroâ€Fenton technique in gas sparged cell. American Journal of Environmental Engineering and Science2, 2(5): 47-59. (2015).
- Beqqal, N., Yahya, M. S., Karbane, M. E. L., Guessous, A., & El Kacemi, K. Kinetic study of the degradation/mineralization of aqueous solutions contaminated with Rosuvastatin drug by Electro-Fenton: Influence of experimental parameters. Journal of Materials and Environmental Sciences, 8(12): 4399-4407. (2017).
- Asghar, A., Salihoudin, A., Aziz Abdul Raman, A., & Mohd Ashri Wan Daud, W. Cathode modification to enhance the performance of inâ€situ fenton oxidation in microbial fuel cells. Environmental Progress & Sustainable Energy, 36(2): 382-393. (2017).
- Soon, A. N., & Hameed, B. H. Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process. Desalination, 269(1): 1-16. (2011).
- Diao, Z. H., Xu, X. R., Jiang, D., Li, G., Liu, J. J., Kong, L. J., & Zuo, L. Z. Enhanced catalytic degradation of ciprofloxacin with FeS2/SiO2 microspheres as heterogeneous Fenton catalyst: Kinetics, reaction pathways and mechanism. Journal of hazardous materials, 327: 108-115. (2017).
- Lin, Z. R., Zhao, L., & Dong, Y. H. Effects of low molecular weight organic acids and fulvic acid on 2, 4, 4'-trichlorobiphenyl degradation and hydroxyl radical formation in a goethite-catalyzed Fenton-like reaction. Chemical Engineering Journal 326: 201-209. (2017).
- Huang, X., Hou, X., Jia, F., Song, F., Zhao, J., & Zhang, L. Ascorbate-Promoted Surface Iron Cycle for Efficient Heterogeneous Fenton Alachlor Degradation with Hematite Nanocrystals. ACS Applied Materials & Interfaces, 9(10): 8751-8758. (2017).
- Pataquiva-Mateus, A. Y., Zea, H. R., & Ramirez, J. H. Degradation of Orange II by Fenton reaction using ilmenite as catalyst. Environmental Science and Pollution Research, 24(7): 6187-6194. (2017).
- Huang, W., Luo, M., Wei, C., Wang, Y., Hanna, K., & Mailhot, G. Enhanced heterogeneous photo-Fenton process modified by magnetite and EDDS: BPA degradation. Environmental Science and Pollution Research, 24(11): 10421-10429. (2017).
- Ganzenko, O., Trellu, C., Papirio, S., Oturan, N., Huguenot, D., van Hullebusch, E. D., Esposito, G., & Oturan, M. A. Bioelectro-Fenton: evaluation of a combined biological—advanced oxidation treatment for pharmaceutical wastewater. Environmental Science and Pollution Research: 1-10. (2017).
- Klein, K., Kivi, A., Dulova, N., Zekker, I., Mölder, E., Tenno, T., Trapido, M., & Tenno, T. A pilot study of three-stage biological–chemical treatment of landfill leachate applying continuous ferric sludge reuse in Fenton-like process. Clean Technologies and Environmental Policy, 19(2): 541-551. (2017).
- Villegas-Guzman, P., Giannakis, S., Rtimi, S., Grandjean, D., Bensimon, M., De Alencastro, L. F., Torres-Palma, R., & Pulgarin, C. A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Applied Catalysis B: Environmental, 219: 538-549. (2017).
- Guo, S., Yuan, N., Zhang, G., & Jimmy, C. Y. Graphene modified iron sludge derived from homogeneous Fenton process as an efficient heterogeneous Fenton catalyst for degradation of organic pollutants. Microporous and Mesoporous Materials, 238: 62-68. (2017).
- Velichkova, F., Delmas, H., Julcour, C., & Koumanova, B. Heterogeneous fenton and photoâ€fenton oxidation for paracetamol removal using iron containing ZSMâ€5 zeolite as catalyst. AIChE Journal, 63(2): 669-679. (2017).
- Mishra, V. S., Mahajani, V. V., & Joshi, J. B. Wet air oxidation. Industrial & Engineering Chemistry Research, 34(1), 2-48. (1995).
- Debellefontaine, H., Chakchouk, M., Foussard, J. N., Tissot, D., & Striolo, P. Treatment of organic aqueous wastes: wet air oxidation and wet peroxide oxidation®. Environmental pollution, 92(2), 155-164. (1996).
- Wellig, B., Lieball, K., & von Rohr, P. R. Operating characteristics of a transpiring-wall SCWO reactor with a hydrothermal flame as internal heat source. The Journal of supercritical fluids, 34(1), 35-50. (2005).
- Zhang, F., & Ma, C. CFD simulation of a transpiringâ€wall SCWO reactor: Formation and optimization of the water film. AIChE Journal, 62(1), 195-206. (2016).
- Rice, S. F., Wu, B. C., Winters, W. S., & Robinson, C. D. Engineering modeling of the pine bluff arsenal supercritical water oxidation reactor (No. SAND2000-8656C). Sandia National Labs., Albuquerque, NM, and Livermore, CA (US). (2000).
- Ahluwalia KS, Crooker P, Meagher GM. Demonstration of transpiring wall SCWO technology for chemical weapons destruction at Blue Grass. Proceedings of the Workshop on Supercritical Water Oxidation—Achievements and Challenges in Commercial Applications, Arlington, VA, Aug. 15; (2001).
- Fauvel, E., Joussot-Dubien, C., Guichardon, P., Charbit, G., Charbit, F., & Sarrade, S. A double-wall reactor for hydrothermal oxidation with supercritical water flow across the inner porous tube. The Journal of supercritical fluids, 28(1), 47-56. (2004).
- Bermejo, M. D., Fdez-Polanco, F., & Cocero, M. J. Effect of the transpiring wall on the behavior of a supercritical water oxidation reactor: modeling and experimental results. Industrial & engineering chemistry research, 45(10), 3438-3446. (2006).







