Growth Versus Storage in Quercus Leucotrichophora And Pinus Roxburghii Seedlings in Responses to Changes In Nutrient and Water Availability
Kiran Bargali1 and S. S. Bargali1
1
Department of Botany,
DSB Campus,
Kumaun University,
Nainital,
263001
Uttarakhand
India
DOI: http://dx.doi.org/10.12944/CWE.10.2.14
In this paper, growth and storage of carbon and nitrogen in seedlings of banj oak (Quercus leucotrichophora A. Camus) and chir pine (Pinus roxburghii Sarg.) have been compared across different levels of nutrient and water availabilities. Four nutrient (144, 264, 384 and 504 mg of NPK fertilizer per kg soil) and three watering (21 days,14 days and 7 days interval) treatments were applied to seedlings. At low watering levels, seedling dry mass of both the species decreased towards higher nutrient level. However, at high watering level, dry mass increased with increasing water availability. When water availability was increased in a constant nutrient environment, dry mass of seedling increased with increase in water availability. Both the species showed a similar pattern of storing nitrogen instead of increasing biomass particularly at low watering levels. However, at each nutrient level, growth and storage increased with increasing moisture availability. As compared to Q. leucotrichophora, seedlings of P. roxburghii favoured growth over storage (according to its more competitive strategy), although this species accumulated more carbon and nitrogen towards the higher nutrient level.
Copy the following to cite this article:
Bargali K, Bargali S. S. Growth Versus Storage in Quercus Leucotrichophora And Pinus Roxburghii Seedlings in Responses to Changes In Nutrient and Water Availability. Curr World Environ 2015;10(2) DOI:http://dx.doi.org/10.12944/CWE.10.2.14
Copy the following to cite this URL:
Bargali K, Bargali S. S. Growth Versus Storage in Quercus Leucotrichophora And Pinus Roxburghii Seedlings in Responses to Changes In Nutrient and Water Availability. Curr World Environ 2015;10(2). Available from: http://www.cwejournal.org/?p=12527
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2015-07-07 |
|---|---|
| Accepted: | 2015-08-06 |
Introduction
During the extensive reforestation programmes, plantations of forest tree seedlings are being promoted in spite of their poor out planting performance. Among, initial seedling size or biomass of forest species has been related to post planting survival (Bargali and Bargali, 1999; Perez et al., 2007) to the ability to out compete other plant species and to the potential for new root production (Jobidon et al., 2003; Bargali and Singh, 1996). In addition, after disturbance, carbon stock is important for both resprouting (Huddle and Pallardy, 1999) and respiration during period of resource shortage (Bargali et al.,1992; Joshi et al.,1997). On the other hand, nitrogen storage affects the rate of growth after planting in the field (Malik and Timmer, 1998) and seedling capacity to recover foliage after disturbances (Bloom et al.,1985).
Seedling biomass, carbon and nitrogen storage may change in response to resource availability but only few studies have addressed integrated response of biomass, carbon and nitrogen storage to resource availability (Perez et al.,2007; Salifu and Timmer, 2003; Bargali and Singh, 1995; Bargali and Singh, 1996). Growth and storage may compete for Carbon and Nitrogen (Chapin et al., 1990; Herms and Mattson, 1992), but resource availability may alter the proportion at which both resources are captured and stored. The aim of the present study was: i) to assess the response of growth, carbon and nitrogen reserve of seedling of Quercus leucotrichophora and Pinus roxburghii to changes in nutrient and water availabilities during their first year of growth; ii) to compare growth versus storage in fast growing early successional P. roxburghii and slow growing late successional Q. leucotrichophora.
Material and Methods
Species
leucotrichophora(Banj oak) and P. roxburghii (Chir pine) are two dominant forest forming tree species of the Central Himalayan region between 1200 to 2200 m elevations. Both are evergreen species with similar periodicities of leafing, leaf fall and leaf longevities being just more than one year (Ralhan et al., 1995). While Q. leucotrichophora is regarded as the major late successional species of the Central Himalayan region (Bargali et al.,2014; 2015), P. roxburghii is referred as early successional species in view of the framework of basic plant strategies provided by Grime (1977).
Experimental Design
The experiments were performed with first year seedlings because this is the plant life stage when selective pressures are stronger (Reich et al., 2003). Seedlings of Q. leucotrichophora and P. roxburghii were raised from the seeds of current year crop and transferred to polyethylene bags (each containing 1 kg prepared soil containing sieved oak forest soil and commercial san in 1:3 ratio). Before starting treatments, ten individuals of each species were separated into their component parts and oven dried to obtain the initial dry mass. The experiment was carried out under glasshouse condition with temperature ranging from 5 oC (minimum) in December- January to 35 oC (maximum) in June.
Fertilization Experiment
Four levels of nutrient were established by adding 144, 264, 384 and 504 mg of 12:32:16 NPK fertilizer to the bags. Hereafter referred to as nutrient level N1, N2, N3 and N4, respectively.
Water stress experiment
Each of the nutrient level was subjected to a gradient of water stress by watering the bags at 21, 14 and 7 day intervals (referred to as W1, W2 and W3 water levels, respectively).
Growth measurements
Ten seedlings per species and treatment were harvested at random at the end of the experiment. Seedlings were separated into leaves, stems and roots. Roots were gently washed to eliminate soil particles. All parts were oven dried at 60o for 48 h and weighed separately.
Chemical analysis
A 0.5 g composite sample of all replicate of a treatment of each component of a species was analysed for total nitrogen, using Kjel Auto VS-KTP Nitrogen analyzer based on micro kjeldhal technique (Peach and Tracey1956, Misra 1968). The nitrogen mass of different component was computed as the products obtained by multiplying dry weight of component with their mean nitrogen concentration. Carbon stock was obtained from conversion of biomass using a conversion coefficient of 0.5 g C g DM-1 (Arora et al., 2011). The effects of treatment and species on biomass, C: N ratio, carbon and nitrogen content of the whole plant and each part were tested by means of a two-way analysis of variance (ANOVA). In addition, treatment effects on whole plant traits were further analysed within each species using a one-way ANOVA.
Results and Discussion
In both the species highest nutrient level failed to increase seedling dry mass (FG1), possibly due to toxic effect of nutrient. Parrish and Bazzaz (1982) also reported death of common early and late successional species at highest nutrient level. In conformity to trend towards the decrease in net primary production (Odum, 1969) and lower net photosynthetic rates (Bazzaz, 1979), as succession proceeds, the maximum production levels were greater in the early successional P. roxburghii than in the late successional Q. leucotrichophora. The responses of both species to nutrient availability were modified by watering frequencies. At each nutrient level, dry mass increased with increasing watering frequencies (FG 1).
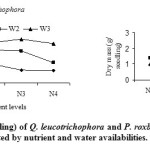 |
|
The ratio of water absorbing surface to transpiring surface (Root: shoot ratio) is probably more important than actual leaf or transpiring surface (FG2). At each nutrient and water level, allocation of mass to roots was greater in Q. leucotrichophora in comparison to early successional P. roxburghii, and a reverse trend for leaf weight ratios are in conformity to previously observed trends (Grime, 1977; Chaudhary, 1989; Bisht, 1990; Bargali, 1992). Root: shoot ratios were generally higher where nutrient and water were limiting factors (i.e. N1W1 level), and lower where these factors were non limiting (N4W3 level). Increased nutrient availability is known to cause a reduction in root: shoot ratio in several plant species (Chaudhary, 1989; Bisht, 1990; Chapin, 1980). Similarly in increase in soil water availability causes a reduction in root: shoot ratios (Bisht, 1990; Rao, 1984; Bargali and Singh, 1995).
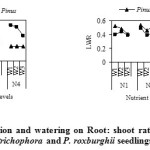 |
|
The carbon stock (g seedling-1) also follow the same trend as described for seedling dry mass (FG3). These results indicate that lower carbon storage in high nutrient and lower water availability would decrease the capacity of seedlings to survive long stress periods or to recover from disturbances that rely on stored carbon (Chapin et al., 1990).
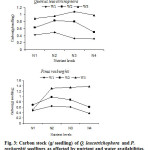 |
|
Total seedling nitrogen mass (g seedling-1) was greater for Q. leucotrichophora than for P. roxburghii at lowest nutrient level, while towards higher nutrient level, nitrogen mass was greater for P. roxburghii (FG4). At low nutrient level P. roxburghii showed growth over storage as indicated by greater dry mass of seedling. Higher nitrogen storage in P. roxburghii seedlings could allow faster growth after transplanting in the field, as nitrogen storage allows faster subsequent growth (Malik and Timmer, 1998; Salifu and Timmer, 2003) and improve ability to recover from defoliating disturbance (Bargali and Bargali, 2000).
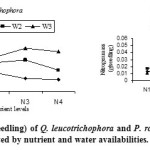 |
|
C: N ratio decreased with increasing nutrient level (FG5), and remains unaffected by water availability. These results suggest that seedling growth was not limited in low nutrient level, and addition of external nutrient supply may promote luxury consumption (Salifu and Jacobs, 2006) and accumulation on nitrogen in plant tissue for future use (Chapin et al.,1990; El Omari et al.,2006; Bargali et al., 2005; Singh et al.,2005).
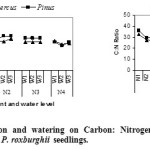 |
|
Acknowledgment
We are thankful to Head, Department of Botany, Kumaun University, Nainital for providing necessary facilities during the research work.
References
- Arora V. P. S., Bargali S. S., Rawat J. S. Climate change: challenges, impacts, and role of biotechnology in mitigation and adaptation. Progressive Agriculture- An International Journal. 2011;11:8-15.
- Bargali K., Joshi B., Bargali S.S., Sing S. P. Diversity within Oaks. International Oaks. 2014;25:57-70.
- Bargali K., Joshi B., Bargali S.S., Singh S. P. Oaks and the Biodiversity They Sustain. International Oaks. 2015;26:65-76.
- Bargali K., Bargali S. S., Singh S. P. Growth and competition between Pinus roxburghii and Quercus leucotrichophora seedlings as affected by nutrient and moisture availability. Bulletin of National Institute Ecology. 2005;15:180-190.
- Bargali K., Bargali S.S. Nutrient utilization efficiencies of two Central Himalayan tree species. Journal of Tropical Forest Science. 2000;12(3):450-458.
- Bargali K., Singh R. P. Effect of light intensity on leaf characteristics of Quercus leucotrichophora seedlings. Acta Botanica Indica. 1996;24:135-137.
- Bargali K., Singh S.P. Competitive abilities of seedlings of an early and a late successional tree species of Central Himalaya grown along a light gradient. Tropical Ecology. 1995;36:237-247.
- Bargali K., Singh S. P. Competitive abilities of Quercus leucotrichophora and Pinus roxburghii seedlings in relation to experiments variations in soil moisture availability. Tropical Ecology. 1996;37: 223-227.
- Bargali K. The responses of Pinus roxburghii and Quercus leucotrichophora to nutrient application. Oecologia Montana. 1992;2:1-6.
- Bargali K., Bargali S. S. Comparative growth response of two contrasting species of Central Himalaya in relation to light and nutrient availability. Journal of Environmental Biology. 1999;20(2):183-187.
- Bargali S. S., Joshi M., Bargali K. Seasonal pattern of total soil respiration in age series of eucalypt plantation and mixed broad-leaved forest in tarai belt of Kumaun Himalaya. Oecologia Montana. 1992;2:7-11.
- Bazzaz F.A. The physiological ecology of plant succession. Annual Review of Ecology and Systamatics. 1979;10:351-371.
- Bisht K. Influence of intraspecific and interspecific competition on Pinus roxburghii and Quercus leucotrichophora along the gradients of soil water nutrient and light. Ph.D. Thesis. Kumaun University, Nainital. 1990.
- Bloom A. J., Chapin F.S.III., Mooney H.A. Resource limitation in plants- an economic analogy. Annual Review of Ecological System. 1985;16:363-392.
- Chapin F. S. III. The mineral nutrition of wild plants. Annual Review of Ecology and Systamatics.1980;11:233-260.
- Chapin F.S.III, Schulze E.D., Mooney H. A. The ecology and economics of storage in plants. Annual Review of Ecological System. 1990;21:423-447.
- Chaudhry S. Ecology of certain pioneer and promising species relevant to recovery of landslide damaged forest sites in Kumaun Himalaya. Ph.D. Thesis. Kumaun University, Nainital. 1989.
- El Omari B., Aranda X., Verdaguer D., Pascual G., Fleck, I. Resource remobilization in Quercus ilex L. resprouts. Plant and Soil. 2003;252:349-357.
- Grime J. P. Evidence for the existence of three primary strategies of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist. 1977;111:1169-1194.
- Herms D. A., Mattson W. J. The dilemma of plants: to growth or defend. Quarterly Review of Biology. 1992;67:283-335.
- Huddle J. S., Pallardy S.G. Effect of fire on survival and growth of Acer rubrum and Quercus seedlings. Forest Ecology Management. 1999;118:49-56.
- Jobidon R., Roy V., Cry G. Net effect of competing vegetation on selected environmental conditions and performance of four spruce seedling stock sizes after eight years in Quebec (Canada). Annals of Forest Science. 60: 691-699 (2003). REFERENCES BARGALI & BARGALI, Curr. World Environ., Vol. 10(2), 494-499 (2015) 499
- Joshi M., Bargali K., Bargali S. S. Changes in physico- chemical properties and metabolic activity of soil in popular plantations replacing natural broad leaved forests. Journal of Arid Environment. 1997;35:161- 169.
- Malik V., Timmer V. R. Biomass partitioning and nitrogen retranslocation in black spruce seedlings on competitive mixed wood sites: a bioassay study. Canadian Journal Botany. 1998;26:1651-1659.
- Misra R. Ecology work book. Oxford and IBH Publishing Company, Calcutta, India. 1968.
- Odum, E. P. The strategy of ecosystem development . Science. 1969;164:262-270.
- Parrish J. A. D., Bazzaz F. A. Responses of plants from three successional communities on a nutrient gradient. Journal of Ecology. 1982;70:233-248.
- Peach, K. and Tracey, M.V. Modern method of plant analysis. Springer Verlag, Berlin, Germany. 1956.
- Perez V. S., Diez P. C., Valladares F. Growth versus storage: responses of Mediterranean oak seedlings to changes in nutrient and water availabilities. Annals of Forest Science. 2007;64:201-210.
- Ralhan P. K., Khanna R. K., Singh S. P., Singh J. S. Phenological characteristics of the tree layer of Kumaun Himalayan forests. Vegetatio. 1985;63:113-119.
- Rao P. B. Regeneration of some trees of western Kumaun Himalaya. Ph.D. Thesis. Kumaun University, Nainital. 1984.
- Reich P. B., Wright I. J., Cavender-Bares J., Craine J., Oleksyn J., Westoby M., Walters M. B. The evolution of plant functional variation: traits, spectra and strategies. International Journal of Plant Science. 2003;164:143-164.
- Salifu K. F., Jacobs D. F. Characterizing fertility targets and multi-element interactiona in nursery culture of Quercus rubra seedlings. Annals of Forest Science. 2006;63:231-237.
- Salifu K. F., Timmer V. R. Nitrogen retranslocation response of young Picea marina to nitrogen-15 supply. Soil Science Society of Amarica Journal. 2003;67:1287-1294.
- Singh S. P., Bargali K., Joshi A., Chaudhry S. Nitrogen resorption in leaves of tree and shrub seedlings in response to increasing soil fertility. Current Science. 2005;89(2):389-396.







