Fuel Property of Biodiesel Made from Microalgae (Chlorella Sp.)
S. V. Kelaiya1 , P. M. Chauhan2 and S. H. Akbari3
1
Department of Bio Energy,
College of FPT and BE,
AAU,
Anand(Gujarat),
India
2
Department of RE and RE,
CAET,
JAU,
Junagadh,
(Gujarat)
India
3
Department of PHET,
College of FPT and BE,
AAU,
Anand (Gujarat)
India
DOI: http://dx.doi.org/10.12944/CWE.10.3.21
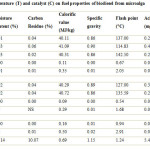
Microalgae chlorella is an organism capable of photosynthesis that is less than 2mm in diameter. The biodiesel extracted from algae using chloroform/methanol extraction solvent system then undergone three different transesterification processes based on three different catalysts viz. Alkali catalyst, Acid catalyst and Enzymatic catalyst with two temperature (50°C and 60 °C) and with 1:5 methanol to bio-oil ratio. After transesterification using different catalysts, the fuel properties were measured. All the properties were compared with standard value of ASTM D 6751 standards. Alkali catalyst yield highest biodiesel (92 %) at 60 °C temperature. Also, the closest value of different fuel properties found at par with standard value of ASTM D 6751 standards viz. moisture content, carbon residue, calorific value, specific gravity, acid value, flash point, viscosity, density, viscosity were found to be 0.01%, 0.04%, 40.41 MJ/kg, 0.83, 0.23 mg KOH/g, 143.67 °C, 5.16 mm2/s, 0.83 g/cm3 respectively in the biodiesel which was yield by transesterification done using Alkali catalyst (0.56 % NaOH) at 60 °C temperature.
Copy the following to cite this article:
Kelaiya S. V, Chauhan P. M, Akbari S. H. Fuel Property of Biodiesel Made from Microalgae (Chlorella Sp.). Curr World Environ 2015;10(3) DOI:http://dx.doi.org/10.12944/CWE.10.3.21
Copy the following to cite this URL:
Kelaiya S. V, Chauhan P. M, Akbari S. H. Fuel Property of Biodiesel Made from Microalgae (Chlorella Sp.). Available from: http://www.cwejournal.org/?p=13184
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2015-09-06 |
|---|---|
| Accepted: | 2015-12-01 |
Introduction
Algae (macro and micro) have been taken in consideration as a residual biomass ready to be used for energy purposes. Algae, especially microalgae, were found to be the only source of renewable biodiesel that is capable of meeting the global demand for transport fuels.1 The idea of using algae as a source of fuel is now being taken seriously because of the increasing price of petroleum and more significantly, the emerging concern about global warming that is associated with burning fossil fuels.4
This work aimed to process development for production of biodiesel from microalgae, using two different extraction solvent systems to get the best result in oil extraction. Biodiesel is produced by trans-esterifying the parent oil or fat with an alcohol, usually methanol, in presence of a catalyst, usually a strong base such as sodium or potassium hydroxide or increasingly, alkoxides, acids and enzymes. The resulting product therefore can contain not only the desired alkyl ester product but also unreached starting material (TAG), residual alcohol, and residual catalyst. Glycerol is formed as by-product and separated from biodiesel in the production process, however, traces thereof can be found in the final biodiesel product. Since transesterification is a stepwise process, MAG and DAG formed as intermediates can also be found in biodiesel.6
With the increasing interest and use, the assurance of fuel properties and quality has become of paramount interest to the successful commercialization and market acceptance of biodiesel. Accordingly, biodiesel standards have been established or are being developed in various countries and regions around the world, including the United States,2 Europe (EN 14214), Brazil, South Africa, Australia and elsewhere.6 In ASTM D 6751 and EN 14214 are commonly used standards as reference or base for other standards, and their analysis.
Materials and Methods
Microalgae (chlorella sp.), which was cultivated from locally available sources which was cultivated for the study. The investigation methodology includes procedure for properties of microalgae biodiesel and analysis of resulting data.
Experimental Design
The experiments were planned using completely randomized design (C.R.D.)5 The treatments consisted three levels of catalyst for transesterification, two levels of temperature and two levels of solvent oil extraction methods in which best suitable method was adopted for oil extraction. The details of treatments and parameters are given as below.
1. Year/Season of experiment: 2013-20142. Crop: Algae, Chlorella Sp.
Experimental Design: C.R.D.
First factor: Catalyst for transesterification(a) Alkali catalyst (C1): Sodium Hydroxide (NaOH)
(b) Acid catalyst (C2): Sulphuric Acid (H2SO4)
(c) Biocatalyst (C3): Lipase Crude
Second factor: Temperature for transesterification(a) 50 °C (T1)
(b) 60 °C (T2)
Third factor: Solvents for oil extraction(a) Chloroform/Methanol (S1)
(b) Hexane/Ether (S2)
Total Treatment combination: 6 and total no of observation (properties) is 8 from each treatment with 3 Replication.
Dependent variable
(a) Quantity of biodiesel
(b) Quality of biodiesel
The results regarding effect of solvent methods on oil recovery and effect of catalyst and temperature on biodiesel recovery were analyzed statistically.5
Extraction Method of Algae oil
In Chloroform-Methanol (2:1, v/v) Method, 10 g of dry algae was taken and mixed with chloroform-methanol (100 ml, 2:1, v/v) for 20 min. using shaker followed by the addition of mixture of chloroform/water (50 ml, 1:1, v/v) for 10 min. Then it was filtered. While in Hexane-Ether (1:1, v/v) method, 10 g of dry algae was mixed with hexane-ether (100 ml, 1:1, v/v); keep it to settle for 24 h3 and then it was filtered.
Transesterification and Biodiesel Production
In this method, oil to methanol ratio was 1:5 and the amount of all three catalysts was 1 % by weight with oil. The reactions were started at two different temperatures likely 50 °C and 60 °C. After reaction took place, biodiesel and glycerin were separated by gravitational method using separating funnel. The produced biodiesel was tested for the different property measurement which are given below,
Analysis of Biodiesel
The fuel properties of algal biodiesel were determined by the following methods.
Moisture Content %
As per ASTM D2709, the moisture content of extracted biodiesel was determined by calculating the loss in weight of sample using hot air oven drying method.
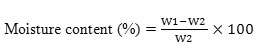
Where,
W1 = Initial weight of biodiesel
W2= Final weight of biodiesel after drying
Carbon Residue %
As per ASTM D4530, percent of carbon residue content was determined. A 10 g of biodiesel was placed in a glass vial and heated to 500°C under an inert (nitrogen) atmosphere in a controlled manner for 1 h. Calculation for the mass % carbon residue in the original sample, or in the 10 % distillation bottoms as follows:
Calculation for percent residue as follows:
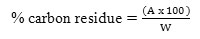
Where,
A = carbon residue, g, and
W = sample used, g
Calorific Value
The calorific value of biodiesel was determined using Bomb calorimeter (ASTM D240). Water equivalent (W) of calorimeter will be obtained by conducting the experiment and using the benzoic acid as standard sample having known calorific value of 'H' equal to 6319 cal/g. The value of W will be calculated by using the following formula.
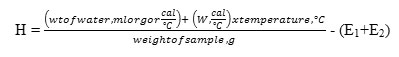
Then, calorific value of biomass will be obtained by conducting the separate experiment by using the following formula and the value of water equivalent, W obtained from the above formula.
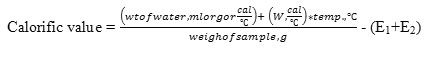
Where,
E1= Correction for nichrome wire, cal= wt of wire, g x calorific value, 335cal/g
E2= Correction for cotton thread, cal=wt of thread, g x calorific value 4180 cal/g
Specific Gravity
As per ASTM D4530, specific gravity of biodiesel was determined by the following equation.
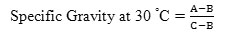
Where,
A = weight in gm of specific gravity bottle with oil at 30°C
B = weight in gm of specific gravity bottle at 30°C
C = weight in gm of specific gravity bottle with water at 30°C
Acid Value
As per ASTM D664, acid value of biodiesel was determined by the following equation.
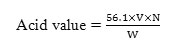
Where,
V = Volume in ml of standard potassium/ Sodium hydroxide
N = Normality of the potassium/ Sodium hydroxide solution
W = Weight in g of the sample
Flash Point
As per ASTM D93, Flash point of biodiesel was determined by Pensky Marten (Closed Cup) method.
Viscosity Measurement of a Liquid
As per ASTM D445, viscosity of biodiesel was determined. The liquids of known densities were allowed to flow through the capillary maintaining the same differences of levels in the limbs and the time equation which governs the flow lead to the relation:
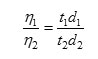
Where, h1 and h2 are viscosity coefficients of the liquid and water, respectively. d1 and d2 are the densities of liquid and water, respectively. Knowing the value of viscosity of one liquid, one can calculate the viscosity of other liquid.
Density at 15 °C
As per ASTM D4052, specific gravity of biodiesel was determined. Density of biodiesel was determined by the following equation.
Density of biodiesel= W/V
Where,
W= weight in gm of biodiesel at 15 °C
V = volume in ml of pycnometer bottle at 15°C
Results and Discussion
Production of Microalgae
Initially in open condition, 20 liters of water was taken in to 200 lit. Capacity tank in which previously prepared culture (4 lit.) and media was added. Whole system was kept for 25 days for the growth of chlorella algae. The rate of production algae was 1.5 g/lit/day. Production data was given in Table 1.
Table 1: Production practice of microalgae
|
Number of days |
Production volume of culture, litre |
Amount of media,g |
Production of wet biomass, g |
Yield of microalgae (dried powder)g |
|
0-25 |
20 |
12.5 |
157 |
36 |
|
26-40 |
60 |
25 |
718 |
158 |
|
41-55 |
80 |
37.5 |
1421 |
341 |
|
56-70 |
100 |
50 |
2020 |
505 |
|
71-85 |
120 |
62.5 |
2741 |
603 |
|
86-100 |
140 |
75 |
3091 |
711 |
|
101-115 |
160 |
87.5 |
3317 |
796 |
|
116-130 |
180 |
100 |
4114 |
905 |
|
131-145 |
200 |
112.5 |
3879 |
1125 |
|
146-160 |
220 |
125 |
5041 |
1210 |
|
Total productivity |
26499 |
6390 |
 |
|
 |
|
Quality Analysis of Algal Bio-Diesel
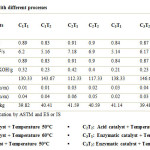
Unlike the previous studies, the present study also evaluated two independent variables: type of catalyst, reaction temperature. Three types of catalyst were used: Alkali catalyst, Acid catalyst and Enzymatic catalyst; two levels of reaction temperature were used: 50 oC and 60 oC. All the experiment combinations were carried out three times in order to determine the consistency of the results and to assess the experimental errors. The statistical data is given in Table 2 and comparison of the properties of algal biodiesel prepared by different methods is given in Table 3.
 |
Figure 3: Biodiesel samples with different treatments Click here to View figure |
 |
|
 |
|
Analysis of Biodiesel
Effect of temperature (T) and catalyst type(C) on different properties of Biodiesel
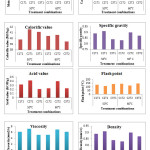
An appraisal data on effect of catalyst type and temperature on different properties of biodiesel is presented in Table 3. Properties like moisture content, carbon residue, calorific value, specific gravity, acid value, flash point, viscosity and density were found to be significant with change in catalyst type. Whereas significant properties with change in temperature were calorific value, specific gravity, acid value, flash point, viscosity and density. As well as the combined effect of the interaction between catalyst and temperature (C x T) on carbon residue was found to be significant while calorific value, specific gravity, acid value, flash point, viscosity and density were found to be highly significant while carbon residue and moisture content were found to be non-significant with change in temperature and the combined effect of the interaction between catalyst and temperature (C x T) on moisture content was found to be non-significant shown in Table 2.
The relationship in between different biodiesel properties and different treatment combinations of biodiesel were shown in Fig. 4.
 |
|
Process Development for Algal Bio-Diesel Production
For the process development of biodiesel from microalgae, represents review of various reaction parameters and other factors which affects the production of algal biodiesel. From all above discussion it has been observed that the reaction parameters which affect the production of microalgae based biodiesel are temperature, catalyst concentrations, physical properties and composition, Purity of Reactants, Mixing Intensity ,type of catalyst used and other factors affects which are Free Fatty Acid (FFA) and moisture level Contains in feedstock. The experiment was conducted for the effect of catalyst type and temperatures and transesterification processes on production of biodiesel from microalgae as described above. As the result shows that Alkali catalyst transesterification process is suitable for the production of biodiesel from microalgae because of the highest biodiesel production and fuel properties of that treatment combination is match with ASTM satandards.
Hence the results obtained from these two methods are depicted in Table 3 and the values of different properties of biodiesel were compared with ASTM standards.
Summary and Conclusion
To develop the process for biodiesel production from microalgae, transesterification of microalgae oil was carried out. For that three different transesterification processes were employed in which three different catalysts, two temperature level and two methods for oil extraction were undertaken.
The results of transesterification process obtained from the study shows that the biodiesel yield was found to be 92 % for alkali catalyst method. Further this sample was analyzed for various fuel properties such as moisture content, carbon residue, calorific value, specific gravity, flash point, acid value, viscosity and density. These properties were compared with ASTM D 6751 standards.
Conclusion
To develop the process for biodiesel production from microalgae, transesterification of microalgae oil was carried out. For that three different transesterification processes were employed in which three different catalysts, two temperature level and two methods for oil extraction were undertaken. The results of transesterification process obtained from the study shows that the biodiesel yield was found to be 92 % for alkali catalyst method. Further this sample was analyzed for various fuel properties such as moisture content, carbon residue, calorific value, specific gravity, flash point, acid value, viscosity and density. These properties were compared with ASTM D 6751 standards. The major conclusions were drawn during the process development of biodiesel from microalgae, as follows.
1. Chlorlla sp., as microalgae for the biodiesel production is used because it can be easily available in fresh water. It also have high oil percentage depends on cultivation practices.
2. The prepared biodiesel was analyzed for different fuel properties viz. moisture content, carbon residue, calorific value, specific gravity, acid value, flash point, viscosity, density, viscosity and their values found to be 0.01%,0.04%, 40.41 MJ/kg, 0.83, , 0.23 mgKOH/g, 143.67 °C, 5.16 mm2 /s, 0.83 g/cm3 respectively. All the properties were found to be closed to the standard value of ASTM D 6751 standards.
3. In the process development for the biodiesel production, the highest yield was obtained in the Alkali catalyst process which was 92 % at the 60 °C temperature and 0.56 % NaOH.
References
- Abd El-Moneim M. R., Emad A. S. and Sanaa M. S. (2010). Enhancement of biodiesel production from different species of algae, grasasy aceites, 61 (4): 416-422.
- ASTM D6751- American Society for Testing Materials.
- Bligh E.G. and Dayer W.J. (1959). A rapid method for total lipid extraction and purification. Canadian Journal of Biochem and Physiol. 37: 911-917.
- Gavrilescu M. and Chisti Y. (2005). Biotechnology a sustainable alternative for chemical industry. Adv. 23: 471-479.
- Gomez K. A. and Gomez A. A. (1984). Statistical procedures for agricultural research. John Wiley & Sons Inc., New York.
- Knothe G. (2006). Analyzing Biodiesel: Standards and other methods. Journal of the American Oil Chemists' Society. 83:823-833.







