Relationship Between Estuarine Shellfish Fauna and Physical Environmental Characteristics for Estuary Conservation in Kyushu, Japan
Rei Itsukushima1 * and Yukihiro Shimatani2
Corresponding author Email: itsukushima@civil.kyushu-u.ac.jp
DOI: http://dx.doi.org/10.12944/CWE.10.3.01
The establishment of a conservation strategy or restoration goal for river estuaries requires knowledge of potential biota or possible habitat characteristics. In this study, we investigated the relationship between estuarine fauna and macro scale physical indicators on Kyushu Island, Japan to provide basic information for estuarine conservation. As a result of the classification of shellfish fauna by similarity, the Kyushu region was divided into three groups, namely, 1) southern Kyushu with high wave exposure, long fetch, and low tidal variation; 2) the Ariake and Yatsushiro seas with low wave exposure, short fetch, and high tidal variation; and 3) northern Kyushu with an intermediate fetch and tidal variation. In addition, a number of sites, such as Nakatsu Port, Sone tideland, and the Honmyou River, were classified into geographically different groups. This is because the physical characteristics of these sites were similar to classified groups or shellfish fauna were significantly altered by artificial impacts. As a result of discriminant analysis, the discrimination hit rate of species inhabiting the inner bay or tidal flat was high, whereas that for species using a wide variety of bottom sediment environment was low. To improve the accuracy of the discriminant model, it is necessary to collect more detailed physical information, such as habitat type, salinity concentration, or grain diameter of bottom sediment. To establish a conservation or restoration strategy, there is a need for classifying taxonomic groups or physical characteristics.
Copy the following to cite this article:
Itsukushima R, Shimatani Y. Relationship Between Estuarine Shellfish Fauna And Physical Environmental Characteristics For Estuary Conservation In Kyushu, Japan. Curr World Environ 2015;10(3) DOI:http://dx.doi.org/10.12944/CWE.10.3.01
Copy the following to cite this URL:
Itsukushima R, Shimatani Y. Relationship Between Estuarine Shellfish Fauna And Physical Environmental Characteristics For Estuary Conservation In Kyushu, Japan. Available from: http://www.cwejournal.org/?p=13247
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2015-11-29 |
|---|---|
| Accepted: | 2015-12-13 |
Introduction
Estuaries are characterized by a spatiotemporally complex environment in terms of both physical and chemical aspects driven by external forces of river flow, tide and ocean waves, or influx of sediment and nutrients.1 Therefore, several definitions of estuaries from the standpoint of physical characteristics or salinity distribution exist.2 In addition, estuaries are important habitats for conserving biodiversity of species that live in only brackish water.3 On the other hand, estuaries have been highly disturbed by human activities since ancient times. The relationship between artificial disturbance and physical and ecosystem response is complicated, because an environmental change in estuaries reflects the impact of the entire upstream river basin. Several studies have investigated impact–response relationships of estuaries. Longphuirt et al. investigated the relationship between land use of the upper basin and nitrogen load.4 Yang et al. measured the geomorphic change in estuaries due to the construction of the Three Gorges Dam.5 Williams et al. studied the influence of estuary weir construction on the sedimentary environment of the river mouth.6 In recent years, studies related to the influence of sea level rise due to climate change have been conducted on the estuary environment.7–9
A conservation strategy based on appropriate understanding of the relationship between physical characteristics and fauna is essential to conserve estuary environments. In Australia, a conservation plan for each estuary has been designed and conservation actions have been progressing. The Australian system classified estuaries by the strength of impact of tide, wave, and river energies.10,11 Estuaries were categorized into six main types (tide-dominated, tide-dominated estuary, tidal flat, wave-dominated delta, wave-dominated estuary, and strandplain), and potential habitat types of each estuary type were suggested.12 Research of relationships between fauna and estuary type13 and reference condition of each estuary type14 were also conducted.
The relationships between physical characteristics and fauna in estuaries have been the subject of past research. One predominant physical indicator is wave exposure. Crisp studied the relationship between wave exposure and barnacles in the English Channel,15 whereas Ebling investigated the distribution of seaweed according to wave action in Lough Hyne (Ireland).16 Concurrently, there has been research on methods to directly measure the degree of wave exposure.17 On the other hand, Ronowicz pointed out that tide is an important physical factor influencing fauna.18
Biota in estuaries vary depending on the physical characteristics of estuaries. Therefore, it is important to elucidate the relationships between estuarine physical characteristics and potential biota to plan estuarine conservation strategies or restoration plans. In the present study, we classified the estuarine fauna of Kyushu Island, Japan and investigated the relationship between the classification result and physical characteristics to obtain basic knowledge for estuarine conservation.
Material and Methods
Analysis objectives
In the present study, we focused on the shellfish (snails and bivalves) inhabiting brackish water. A number of studies using brackish fish fauna to evaluate the estuarine environment exist.19,20 However, brackish water fishes are not necessarily appropriate indicator species for evaluating the estuarine environment because they are not necessarily confined to the brackish water environment. In contrast, shellfishes are relatively sessile and are, therefore, captive within brackish estuarine environments and are affected by water quality or bottom sediments to a greater degree. In addition, some shellfish species are specialists and can only survive in a particular environment. Therefore, shellfish species reflect the current state of the habitat directly and are themost relevant organisms for evaluating the ecological health of a habitat.21
Biological data and analysis method
The shellfish data presented in Table 1 were obtained from the National Census on River Environments from the Ministry of Land, Infrastructure, Transport and Tourism (MLIT) that was conducted from 1993 to 2010 and the National Survey on the Natural Environment by the Ministry of Environment (MOE) was conducted during 2007. Species highlighted in black are important from the point of view of conservation. We used presence–absence data for each species for analysis. The Kikuchi River and the Onga River (MOE and MlIT survey points) were regarded as the same spatial point due to their close spatial proximity. The locations of sampling points are shown in Figure 1.
 |
|
 |
|
We conducted a Two-Way Indicator Species Analysis (TWINSPAN)22 for classifying shellfish fauna based on the similarity of shellfish fauna at each site. PC-ORD version 4 (MjM software design) was used to calculate TWINSPAN. The pseudo species cut-off level was defined as 0 (i.e., presence–absence), and the maximum number of indicator species for a division was set to five.
Physical Indicators
The composition of estuarine biota varies, depending on tide, wave action, and stream flow. In the current study, we adopted wave exposure and direct fetch as indicators of wave action and adopted tidal variation as an indicator of sea tide. Wave exposure was quantified by the Baardseth Index.23 To calculate the index, the center of a transparent circular disc with a radius of 15 cm (representing 7.5 km) was placed on the investigation site on a 1:50 000 chart. The disc was divided into 40 sectors, with the angle of each sector being 9°. Sectors containing peninsula, islands, or parts of the mainland shore were ignored.24,25 We calculated direct fetch by integrating the distance to the opposite shore from the investigation point, extending the radius in each direction with a width of ±22.5° from the center point.26 A maximum distance of 200 km was set as the distance to the opposite shore in accordance with the concept of wave height saturation.27 Tidal variation was calculated by the average of the difference of monthly maximum and minimum tide level for the period January–December 2014. The tidal data were obtained from the Japanese Coast Guard (http://www1.kaiho.mlit.go.jp/KANKYO/TIDE/tide_pred/). The tide of the investigation point was adopted from the recorded value of the nearest Japanese Coast Guard observation point.
Results
Results of shellfish classification
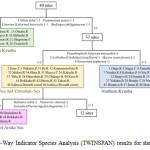
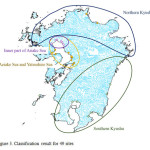
Figure 2 represents the result of TWINSPAN for shellfish fauna at 49 sites. At the first level, 49 sites were classified into 12 rivers situated within southern Kyushu (including the Gokase, Omaru, and the Mannose rivers), and the remaining 37 sites were classified by the presence of Clithonfaba, Psammotaea minor, Littorina (Littorina) brevicula, and Ruditapes philippinarum. At the second level, 37 sites were classified into 17 sites within the Ariake and Yatsushiro seas (including the Midori, Shira, and Kikuchi rivers) and the 20 sites situated within northern Kyushu such as the Onga and the Yamakuni rivers by the presence of Pseudomphalalatericeamiyazakii. The sites situated within the Ariake and Yatsushiro seas were further classified into the inner region of the Ariake Sea (the Yabe, Chikugo, Rokkaku, Okihata, and Shiota rivers) and other sites. The presence of Salinatortakii, Nassariussinarus, and Stenothyra sp. were confirmed in the inner region of the Ariake Sea. In addition, the Honmyou River is geographically situated within the Ariake Sea; however, it is classified into the other groups. In contrast, Sone tideland and Nakatsu Port are geographically situated within northern Kyushu; however, they are classified into the Ariake and Yatsushiro seas group. Figure 3 shows these classification results.
 |
|
 |
|
Relationship between classification result and physical indicators
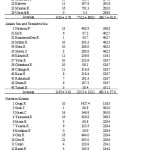
Table 2 lists the classification result as well as the physical indicator values. Wave exposure and direct fetch of the southern Kyushu group was the largest, whereas tidal variation of this group was the smallest among the three groups (wave exposure: 9.83 ± 5.78, direct fetch: 773.2 ± 808.5, tidal variation 240.7 ± 41.6). Most of the sites within this group were facing open sea with a high degree of exposure. In the Ariake and Yatsushiro seas group, the value of direct fetch (215.5 ± 122.1) was the smallest, whereas tidal variation was the largest (483.5 ± 57.0) among the three groups. Most sites belonging to this group were located in the inner bay and were greatly affected by tide. In addition, the value of wave exposure of the Ariake and Yatsushiro seas group was intermediate to that of the northern and the southern Kyushu groups. The value of wave exposure of the northern Kyushu group was the smallest (6.26 ± 6.88), whereas direct fetch (298.2 ± 407.0) and tidal variation (314.0 ± 114.5) was intermediate between the other two groups. The standard deviations of these physical indicators in northern Kyushu were high. Therefore, these groups appear to include a wide variety of environments.
 |
|
Discussion
Similarity Index of each Group
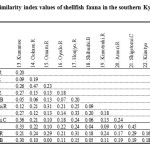
The similarity of the shellfish fauna in each group is discussed here using Jaccard’s Index.28 Table 3 shows the similarity index of shellfish fauna in the southern Kyushu group. The highest index value was obtained between the Oyodo and the Gokase rivers (0.47), both facing the Hyuga-Nada Sea. The second highest index value was obtained between Kiiretyo and the Shigetomi Coast (0.45), falling within Kagoshima Bay. Urauchi Bay situated on Koshiki Island showed a low similarity to other sites (0.10 ± 0.08), and there were no common species within the Omaru River situated within the Hyuga-Nada sea region. In addition, Shibushi Bay, which is characterized by poor diversity of shellfish fauna, presented low similarity to the other sites.
 |
|
Table 4 indicates the similarity index of shellfish fauna in the Ariake and Yatsushiro seas group. The average similarity index (0.25 ± 0.12) was the highest among the three groups, and an even higher index value was indicated for the inner region of Ariake Sea (0.34 ± 0.13). Furthermore, the similarity index of the adjacent estuary of the Yabe and the Chikugo rivers, both belonging to the inner region of the Ariake Sea, was the highest in this group. This is because the inner part of Ariake Sea occurs within an enclosed bay, and therefore, endemic shellfish fauna were formed. The similarity index between the Sone tideland, Nakatsu Port, and the Kumamoto Ono River was high, although these three sites are separated by a long distance. The characteristics of physical indicators of Sone tideland and the Kumamoto Ono River were a low direct fetch and a high tide variation (Table 2). In addition, Nakatsu Port is affected by large tide variation. Since the physical indicators of these three sites are similar, it is believed that shellfish fauna are also similar due to responding to physical characteristics. On the other hand, the similarity index to the other sites of Isahaya Bay south and north coast are low as Ellobiumchinense, Cerithidea (Certhidea) largillierti, and Cerithidea (Cerithidea) ornate, which are typical species in Ariake Bay, were not confirmed at these two sites. These species are likely to have died out at these two sites due to the closure of draining Isahaya Bay. Sato reported on a dramatic change in shellfish faunal composition after the closure of draining Isahaya Bay.29 The results of the current study support their findings.
 |
|
Table 5 shows the similarity index values of shellfish fauna in the northern Kyushu group. The average similarity of the sites was 0.21 ± 0.10. The values of the index in Beppu Bay (the Oita, Ono, and Yasaka rivers, Kofukae Port, and Morie Bay) are high. In particular, the similarity index value of the adjacent estuary of the Oita River and the Ono River is the highest in this group (0.48). On the other hand, shellfish fauna of Yokaku Bay, Yamakuni River, and Imari Bay are similar, although these three sites are separated by a long distance. These sites are likely to show similar physical characteristic. The Honmyou River was classified into the North Kyushu group, although it is geographically positioned in Ariake Bay. In these sites, only five species were confirmed and the similarity index to other sites was low. It is assumed that closure of draining Isahaya Bay influenced the shellfish fauna of Honmyou River in the same way as Isahaya Bay south and north coast. In addition, Namako Pond located on Kosiki Island showed a low similarity to the other sites. This is because this is the only site which is a brackish lake, and a differential environment is formed as compared with the other sites.
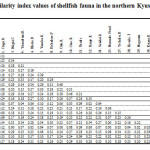 |
|
Discriminant analysis for shellfish species
We developed a prediction model of occurrence using physical indicators by discriminant analysis for 34 species, which were confirmed at over 30 sites. Discriminant analysis was conducted based on Mahalanobis-generalized distance. Statistical analysis software R was used for all analyses.
Table 6 shows the results of discriminant analysis. The linear discrimination factor that indicates how a factor contributes to the prediction for the presence or absence of objective species is showed in the column of the physical indicators in Table 6. In addition, we assembled the habitat information of each species using various literature sources. The discrimination hit rates of Pseudomphalalatericeamiyazakii and Cerithidea (Certhidea) largillierti that inhabit the inland bay or tidal flat were high. This revealed that prediction of the presence or absence of these species is to some extent possible using tidal variation, direct fetch, and wave exposure. On the other hand, the presence of Solenstrictus that use a relatively wide range of bottom sediment environment, Littorina (Littorina) brevicula that adhere to reef, or Clithonretropictus that inhabit the low salt density area are difficult to predict using this model. It is believed that the physical indicators used in this model (direct fetch, wave exposure, and tidal variation) are macro scale; therefore, habitat scale information, such as salinity, habitat, or bottom sediment, is necessary to predict the presence of shellfishes. It is possible to improve prediction accuracy using quantitative data of habitat scale.
 |
|
There is also a need for estuary typology from the perspective of the physical environment. Classifying estuary type from a physical and ecological perspective is essential to develop protection strategies or restoration plans for estuaries. Integrating this information will assist in deciding principle species for conservation or restoration by simple field surveys.
Conclusion
The objective of the current study was to classify shellfish fauna and explore relationships between faunal composition and physical characteristics within the Kyushu Region, Japan. The acquired knowledge obtained from the results of the current study includes the following:
1. As a result of classifying shellfish fauna, the estuary of Kyushu Island was divided into three groups (southern Kyushu, Ariake and Yatsushiro seas, and northern Kyushu). Physical characteristics of each group are mentioned below.
- Southern Kyushu: high wave exposure, long fetch, and low tidal variation.
- Ariake and Yatsushiro seas: low wave exposure, short fetch, and high tidal variation.
- Northern Kyushu: intermediate values of fetch and tidal variation.
2. A number of sites, such as Nakatsu Port, Sone tideland, and Honmyou River, were classified into geographically different groups. This is because physical characteristics of these sites were similar to classified groups, or shellfish fauna was significantly altered by artificial impact.
3. As a result of discriminant analysis, the discrimination hit rate of species inhabiting the inner bay or tidal flat was high, whereas the hit rate was low for species using a wide variety of bottom sediment environment. To improve the accuracy of the discriminant model, it is necessary to collect more detailed physical information, such as habitat type, salinity concentration, or grain diameter of bottom sediment.
In the present study, we classified shellfish fauna because of the strong relationship between fauna and the physical environmental. To establish conservation or restoration plans, there is need for classifying other taxonomic groups or physical characteristics.
Acknowledgment
This work was supported by JSPS KAKENHI Grant Number 15K18144. The authors would like to thank Nature Conservation Bureau, Ministry of the Environment of Japan for supplying rare species data.
References
- McLusky, D. S. The Estuary Ecosystem, Chapman and Hall, 2nd edition (1989).
- Dyer, K. R. Estuaries A Physical Introduction.Wiley (1997).
- Kusuda, T., and Yamamoto, K. (2008) River Brackish area. GihodoShuppan 353(in Japanese).
- Ní Longphuirt, S., O'Boyle, S., and Stengel, D. B. Environmental Response of an Irish Estuary to Changing Land Management Practices. Science of the Total Environment, 521-522: 388–399 (2015).
- Yang, S.L., Milliman, J. D., Xu, K. H., Deng, B., Zhang, X. Y., and Luo, X. X. Downstream Sedimentary and Geomorphic Impacts of the Three Gorges Dam on the Yangtze River. Earth-Science Reviews, 138(1): 469–486 (2014).
- Williams, J., Dellapenna, T.,Lee, G. H., and Louchouarn, P. Sedimentary Impacts of Anthropogenic Alternations on the Yeongsan Estuary, South Korea. Marine Geology, 357(1): 256–271 (2014).
- Schlenk, D., and Lavado, R. Impacts of Climate Change on Hypersaline Conditions of Estuaries and Xenobiotic Toxicity. Aquatic Toxicology, 105(3-4): 78–82 (2011).
- James, N. C., van Niekerk, L., Whitfield, A. K., Potts, W. M., Götz, A., and Paterson, A. W. Effects of Climate Change on South African Estuaries and Associated Fish Species. Climate Research, 57(3): 233–248 (2013).
- Peirson, W., Davey, E., Jones, A., Hadwen, W., Bishop, K., Beger, M., Capon, S., Fairweather, P., Creese, Smith, T. F., Gray, L., and Tomlinson, R. Opportunistic Management of Estuaries under Climate Change: A new adaptive decision-making framework and its practical application. Journal of Environmental Management, 163(1):214–223 (2015).
- Dalrymple, R. W., Zaitlin, B. A., and Boyd, R. Estuarine Facies Models: Conceptual Models and Stratigraphic Implications. Journal of Sedimentary Petrology, 62:1130–1146 (1992).
- Boyd, R., Dalrymple, R. W., and Zaitlin, B. A. Classification of Clastic Coastal Depositional Environments. Sedimentary Geology, 80: 139–150 (1992).
- Heap, A., Bryce, S., Ryan, D., Redke, L., Smith, R., Harris, P., and Heggie, D. Australian Estuaries and Coastal Waterways: A Perspective for Improved and Integrated Resource Management. Australian Geological Survey Organization, Record 2001/07 (2001).
- Harrison, T. V., and Whitfield, A.K. Estuarine Typology and the Structuring Of Fish Communities in South Africa. Environmental Biology of Fishes, 75: 269–293 (2006).
- Fairweather, P. G. Determining the ‘Health’ of Estuaries: Priorities for Ecological Research. Australian journal of ecology, 24(4): 441–451 (1999).
- Crisp, D. J., and Southward, A. J.The Distribution of Intertidal Organisms along the Coasts of the English Channel. Journal of the Marine Biological Assessment of the United Kingdom, 37(1): 157–203 (1958).
- Ebling, F. J., Sleeigh, M. A., Sloane, J. F., and Kitching, J. A. The Ecology of Lough Ine: VII. Distribution of Some Common Plants and Animals of the Littoral and Shallow Sublittoral Regions. Journal of Ecology, 48(1): 29–53 (1960).
- Palumbi, S. R. Measuring Intertidal Wave Forces. Journal of Experimental Marine Biology and Ecology, 81(2):171–179 (1984) (in Japanese).
- Ronowicz, M. Species Diversity of Arctic Gravel Beach: Case Study for Species Poor Habitats. Polish Polar Research, 26(4): 287–297 (2005).
- Inui, R., Nishida, T., Onikura, N., Eguchi, K.,Kawagishi, M., Nakatani, M., and Oikawa, S. Physical Factors Influencing Immature-Fish Communities in the Surf Zones of Sandy Beaches in Northwestern Kyushu Island, Japan. Estuarine, Coastal and Shelf Science, 86(3): 467–476 (2010).
- Koyama, A., Inui, R., Iyooka, H., Akamatsu, Y., and Onikura, N. Habitat Suitability of Eight Threatened Gobies Inhabiting Tidal Flats in Temperate Estuaries: Model Developments in the Estuary of the Kuma River in Kyushu Island, Japan. Ichthyological Research, 19 (2015).
- Sato, S. Report on Four Academic Societies Joint Symposium of Biodiversity Conservation of Ariake Bay. Japanese Journal of Benthology, 66(2): 102–116 (2011) (in Japanese).
- Hill, M. O. TWINSPAN: A FORTRAN Program for Arranging Multivariate Data in an Ordered Two-Way Table by Classification of the Individuals and Attributes. Ecological and Systematics Department. Cornell University, New York (1979).
- Baardseth, E. A Square Scanning, Two Stage Sampling Method of Estimating Sea Weed Quantities. Norskinstitutt for tang-ogtareforskning,Rapport,33: 1–41 (1970).
- Ruuskanen, A., Bäck, S., and Reitalu, T. A Comparison of Two Cartographic Exposure Methods Using Fucusvesiculosus as an Indicator. Marine Biology, 134(1): 139–145 (1999).
- Ogaki, S. Geological Method for Exposure Measurement. Argonauta, 16: 25–38 (2009) (In Japanese).
- Keddy, P. A. Quantifying a within-lake gradient of wave energy in Gillfillan Lake, Nova Scotia, Canadian Journal of Botany, 62(2): 301–309 (1982).
- Burrows, M., Harvey, R., and Robb, L. Wave Exposure Indices from Digital Coastlines and the Prediction of Rocky Shore Community Structure. Marine Ecology Progress Series, 353:1–12 (2008).
- Jacccard, P. The Distribution of the Flora in the Alpine Zone. New Phytologist,11: 37–50 (1912).
- Sato, S., Azuma, M., Kondo, H., and Nishinokubi, K. Spatial Distributions of Bivalves and Gastropods on the Tidal Mud Flat Created by Construction of a Dike for Reclamation of Isahaya Bay, Western Kyushu, Japan. The Quaternary Research, 40(1): 43–51 (2001).
- Sassa, S., Watanabe, Y., and Yang, S. Performance and Mechanics of Self-burial Activities of Bivalves, Japanese Littleneck. Proceedings of the Japanese conference on coastal engineering, 65(1): 1116–1120 (2009).
- Wada, K., Nishihira, M., Furota, T., Nojima, S., Yamanishi, R., Nishikawa, T., Goshima, S., Suzuki, T., Kato, M., Shimamura, K., and Fukuda, H. Present Status of Estuarine Locales and Benthic Invertebrates Occurring in Estuarine Environment in Japan. WWF Japan Science Report, 3: 1–182 (1996) (in Japanese).
- Matsuo, K., Masuda, T., Misonou, T., Igarashi, M., Morimoto, K., and Takikawa, K. Study on Indicator Species of Tidal Flat in Ariake bay. Journal of Japan Society of Civil Engineering, Ser. B2, Coastal engineering, 67(2): I_481– I_486 (2011) (In Japanese).
- Tomita, Y., and Tomiyama, K. Life history of Nassariusfestiva (Powys, 1833) (Gastropoda: Nassariidae) on a Mangrove Tidal Flat in Kiire, Kagoshima, Japan. Nature of Kagoshima, 40: 155–158 (2014) (in Japanese).
- Miura, T. A Field Guide to Animals of Tidal Flats. Nanpou-shinsha, Kagoshima (2008) (in Japanese).
- Ito, K., Sasaki, K., Omori, M., and Okata, A. Growth Rate of the Bivalve Nuttalliaolivacea Measured by Marking-recapture and Caging. Japanese Journal of Benthology, 56: 9–17 (2001) (in Japanese).
- Wada, T. The Macrobenthic Fauna in Yoshino River Tidal Flat, with Discussion on Conservation of Estuary Biodiversity (survey in 2011-2012). Bulletin Tokushima Prefecture museum, 23: 87–111 (2013) (in Japanese).
- Yamashita, H., Seino, S., Uda, T., Mori, H., Kudo, H., Nakashima, A., and Ehira, Y. Relationship between Longitudinal Salinity Gradient and Habitat Distribution of Freshwater, Estuarine and Marine Mollusks in the Lower Yasaka River. Annual journal of hydraulic engineering, JSCE, 46: 1187–1192 (2002) (in Japanese).
- Adachi, N., and Wada, K. Distribution of Two Intertidal Gastropods, Batillariamultiformis and B. cumingi (Batillariidae) at a Co-occurring Area.Venus: the Japanese journal of malacology, 57(2): 115–120 (1998) (in Japanese).
- Okutani, T. Marine Mollusks in Japan.Tokai University Press (2000) (in Japanese).
- Kimura, T. Why an Endangered Snail Ellobiumchinense Aggregates on the Uppermost Tidal Flat? Bulletin of the Japanese Society of Scientific Fisheries, 77(1): 119 (2011) (in Japanese).
- Iijima, A.,Kurozumi, T., and Furota, T. A Reestablishment of Cerithideadjadjariensis (Martin) (Mollusca, Gastropoda) Population, a Endangered Tidal Flat Snail, on an Artificial Tidal Flat at the Innermost of Tokyo Bay. Japanese Journal of benthology, 57: 34–37 (2002) (in Japanese).
- Kohama, T., Montani, S., Kajiwara, Y., and Yamada, M. Population Dynamics of Sessile Bivalves Mytilusgalloprovincialis and Xenostrobussecuris in Hyper Eutrophicated Bay, Japan. The Japanese Society of Fisheries Science, 67(4): 664–671 (2001) (in Japanese).
- Horikoshi, A., and Okamoto, K.Present Sessile Organism Community Structure on the Intertidal Coast of Tokyo Bay. Sessile Organisms, 24(1): 9–19 (2007) (in Japanese).
- Nishi, E. and Tanaka, K. Benthos Fauna of Tamagawa River Estuary. Natural History Report Kanagawa, 27; 77–80 (2006) (in Japanese).
- Yajima, T. Studies on the Intertidal Communities of the Sea of Japan IV. General Features of the Zonation of Rocky Shores in Oga Peninsula, Bulletin of the Japan Sea Research Institute, Kanazawa University, 12: 1–17 (1980) (in Japanese).
- Sato, K. Auriculastraduplicata in the Ariake Sea: a running population. The malacological society of Japan, 11(4): 72–73 (1980) (in Japanese).
- Takada, Y., Abe, O., and Shibuno, T. Long-term Variation in a Population of Patelloidaheroldi on a Boulder Shore (Abstracts of Papers Presented at the 2006 Annual Meeting of the Malacological Society of Japan (Tokyo)), Venus: the Japanese journal of malacology, 65(3): 228 (2006)(in Japanese).
- Eiko, M., Ohtaki, H., and Tomiyama, K. Distribution and Substrate Preference among Four Batillarid and Patamidid Species, with Observations on Seasonal Changes in the Distribution of Cerithideopsilladjadjariensis (K. Martin, 1889) (Gastropoda: Potamididae). Venus: the Japanese Journal of Malacology, 61(1-2): 61–76 (2002) (in Japanese).
- Yamamoto, Y., and Wada, K. Distribution Characteristics of Cerithideacingulata in Tidal Flat. NankiSeibutsu, 41(1): 15–22 (1999) (in Japanese).
- Otani, S., Kozuki, Y., and Yamanaka, R. Distribution Characteristics and Population Dynamics of Cerithidae cingulate in Tidal Flat of River Mouth. Journal of Japan Society of Civil Engineering, Ser.B3, Coastal engineering, 67(2): 487–492 (2011) (in Japanese).
- Koga, T., and Matuura, S. Distribution and Size Composition of Batillariamultiformis and Cerithidea Cingulate in Tidal Flat of Wakayama NankiSeibutsu, 45(2): 85–91 (2003) (in Japanese).







