Removal of BOD5 and COD From Saline Wastewater Using Fixed Bed Column of Aspergillus Oryzae and Halobacillus Dabanensis
Sara Ghaed1 * , Reza Marandi2 and Flor Mazhar3
Corresponding author Email: saraghaed308@yahoo.com
DOI: http://dx.doi.org/10.12944/CWE.10.3.14
5-day BOD and COD can be removed by biological aerobic treatment of saline wastewater. In this research, halophilic microorganisms, namely Aspergillus oryzae and Halobacillus dabanensis were isolated from a return sludge basin of a wastewater treatment plant in the City of Bandar Abbas in southern Iran , that contained a Total Dissolved Solid (TDS) of about 7500 mg l-1. These microorganisms (bacteria and fungi) could tolerate 20% concentration of salt (NaCl) in Sabouraud-4% dextrose agar and Sabouraud-2% dextrose broth medium and brain heart (BHI) agar and BHI broth medium. The films of Aspergillus oryzae and Halobacillus dabanensis were formed around the Ca-alginate. These bioflims were introduced to a fixed bed column, on top of which saline wastewater was released with flow rates of 2-6 ml min-1. According to the results of Stover-Kincannon model, the constant values of maximum BOD and COD were estimated at 0.066 mg BOD5 l-1min-1 and 0.1449 mg COD l-1 min-1, respectively. The saturation constant values, at the flow rate of 2ml min-1, by Aspegillus oryzae were 0.00003 mg BOD5 l-1min-1 and 0.00038 mg COD l-1min-1. The removal process in fixed bed column was stopped after 1200 minutes.
Copy the following to cite this article:
Ghaed S, Marandi R, Mazhar F. Removal of BOD5 and COD From Saline Wastewater Using Fixed Bed Column of Aspergillus Oryzae and Halobacillus Dabanensis. Curr World Environ 2015;10(3) DOI:http://dx.doi.org/10.12944/CWE.10.3.14
Copy the following to cite this URL:
Ghaed S, Marandi R, Mazhar F. Removal of BOD5 and COD From Saline Wastewater Using Fixed Bed Column of Aspergillus Oryzae and Halobacillus Dabanensis. Curr World Environ 2015;10(3). Available from: http://www.cwejournal.org/?p=12893
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2015-04-26 |
|---|---|
| Accepted: | 2015-09-12 |
Introduction
Industries are responsible for the release of billion gallons of wastewater containing high levels of salt and organic matters1 into the environment. Such a huge amount of waste generated in the world is capable of being reused as a source of water supply in areas with water shortages.2 More efficient treatment methods should be utilized before reusing and/or discharging the saline wastewater to the environment.3
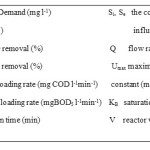 |
Table Click here to View table |
Salinity is known to have toxic influences on bacteria and is also capable of altering the microbial community.4 It can affect the metabolism of microorganisms.5 Excessive salt content in wastewater inhibits large number of enzymes and causes salt stress among microbial species.6, 7
This can decrease biological7 and / or cellular activities and ultimately leads to plasmolysis.6 It would be a rather high-priced procedure to remove salinity by physicochemical process in advance of biological treatment [8]. Regardless of being inconvenient, bio-treatment of saline wastewater is cost-effective and environment friendly.5 Efficiency of bio-treatment of saline wastewater is poor especially for the removal of COD. This is mainly because of negative impact of salt on microbial flora and augmentation of suspended solids in the effluent mainly at high concentration of salt (>2%).9,10 Sudden salinity shift affects adversely the bio-treatment process more than fluctuation within a limited range, which widely inhibits aerobic bio-treatment.3 According to the literature, aerobic treatment is affected negatively in case chloride exceeds 5000-8000 mg l-1.12 Halophilic bacteria have been recommended by some researchers for bio-treatment of saline wastewater.13 Sequencing Batch Reactor (SBR) is a satisfactory system for bio-treatment at high salinity, aerobic biosolids have poor settlement due to effluent turbidity, and membrane coupled bioreactor including Ultra Filtration (UF) or Micro-Filtration (MF).5
Some halophilic microbes are able to improve treatment within a wide range of salinity and are adaptable to salinity shock.11 Salinity tolerance is the primary way to enhance efficiency of bio-treatment of hypersaline wastewater. It should be mentioned that microbes could grow at the salinity range of 0-15% while halophilics tolerate grow well at the salinity range of 1-30%.11 Accordingly, halophilics survive at high salinity.14
The microbial structure of aerobic granules is denser and thus they settle rapidly, and furthermore they could bear the incoming shocks and are tolerant of medium toxic environments.15 Extracellular Polymeric Substances (EPS) are a combination of polysaccharides, nucleic acids and lipids.16
Immobilization may be an impressive method to inhibit biomass from being washed out; when environmental factors are not helpful, or there are available toxic substrates are present.17 The feasible influence of salt on immobilized biomass has given less notice so far due to different studies utilizing disparate operating situations and microbial communities.18
According to the report, high concentrations of salt (NaCl) can decrease membrane penetrability.5 Reid et al. used an immersed Membrane Bioreactor (MBR) to study the impacts of salinity shocks (up to 5000 mg l-1) on qualifications of activated sludge as well as the penetrability of membrane.19 Sun et al.20 investigated the impacts of salinity at deferent salt concentrations of (1000 mg l-1, 2500 mg l-1, 5000 mg l-1, 15,000 g l-1NaCl) on a biofilm-MBR process for shipboard wastewater treatment. Results demonstrated that the membrane fouling rates between 0.45 mbar day-1 and 0.47 mbar day-1 for 0 mg l-1 and 1000 mg l-1salt, respectively [20]. High salinity effects on nitrification and denitrification have already been studied.21 However, the mechanism of aerobic granulation in treatment of saline wastewater is still unknown.7 Bio-treatment of hypersaline wastewater by pure halophilic bacteria has been investigated in various biofilms and SBR.14,22
In this research, a fixed bed reactor containing biofilm of fungi and bacteria species was used for removal of BOD5 and COD from saline wastewater. The fungi and bacteria species reused from a municipal wastewater treatment plant located in Bandar Abbas City, southern Iran. The preferences of an aerobic fixed bed include (i) stubby Hydraulic Retention Time (HRT), (ii) high specific surface area, (iv) in sensitivity to toxic substrate, low energy consumption, and (vii) simplicity of operation.
Materials and Methods
Optimizing growth conditions of fungi and bacteria
In this study, the returned activated sludge a municipal wastewater treatment plant in Bandar Abbas City was sampled for purification of fungi and bacteria species according to the relevant standard methods. The species of fungi and bacteria were identified by Polymerase Chain Reaction (PCR). The species were cultured in Sabouraud-4% dextrose agar (Merck) and brain heart (BHI) agar (Merck) medium with 5 to 25% NaCl concentrations to determine the tolerance limit of the fungi and bacteria to medium salinity. Temperature range for the growth of fungi and bacteria species was set between 28- 50ºC in order to obtain the optimized temperature. Fungi and bacteria species were used for the formation of biofilm around the Ca-alginate bed and treatment of the saline wastewater.
Formation of biofilm
10 g of sodium alginate powder was added to 50-60 times that of distilled water. To make it humanized, the mixture was heated by a heater. Then, the solution was poured into a 0.2 M solution of CaCl2 to form Ca-alginate granules. 23 The Ca-alginate granules have to be rotating during the formation. The solution was placed in a refrigerator for 24 h to make the granules stronger. The Ca- alginate granules were poured in the sterile Sabouraud-2% dextrose broth (Merck) and BHI broth (Merck) medium; and autoclaved. Fungi and bacteria species were inoculated into Sabouraud-2% dextrose broth and BHI broth medium based on the optimized amounts of inoculation. It is worth mentioning that the optimum inoculation amounts of fungi and bacteria species were calculated based on the dry mass and Mcfarland standards,24 respectively. They were placed in a shaker with a rotation speed of 150 rpm at the optimized temperature. This process was studied with Scanning Electron Microscopy (SEM) image in order to make sure of the formation of fungi and bacteria films around the Ca- alginate.
Fixed bed column
The fixed bed reactor was in the form of a cylindrical column; 50 cm in height and 4.5 cm in diameter. It was made of glass (Fig.1). The wastewater in the fixed bed column was taken from the Grit Chamber. Saline wastewater was filtered through filter papers of Whatman GF-Ctype to prevent blockage of the column due to entry of suspended particles and colloids. After the biofilms (fungi and bacteria) were formed around the Ca-alginate; was added to the column. It was aerated from the bottom by an aquarium pump. In order to adapt the environmental conditions, the fungi and bacteria films were settled in the column, respectively for 43 h and 24 h, to adapt to the conditions of the environment. Meanwhile, the brownish light-green pigments were formed in the fungi biofilms. Omil et al. (1995) showed it is possible to adopt an active methanogenic biomass at the salinity content as same as the effluent. They concluded that the process performance depends on appropriate strategies for adaptation of the biomass to high salinity.25
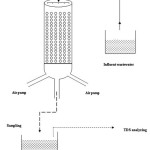 |
Figure 1 Click here to View figure |
After the period of 43 and 24 h, the municipal wastewater with concentrations of TDS 7500 mg l-1 and the flow rates of 2, 4 and 6 ml min-1 was conducted to the column from top. The amounts of COD and BOD5 were measured every 24 h.26 The pH of saline wastewater was measured with HORIBA model pH meter (F-12) and was found to be 8 based on batch operation. The pH was controlled every day.
Kinetic Model
The kinetics of substrate (COD and BOD5) elimination in the bioreactor was estimated a modified Stover–Kincannon model that, at a steady state, has the following form27:
ds/dt=(Umax)Q.Si)/V)/(KB+(Q.Si)/V) (1)
Form (1) may be liberalized as
(ds/dt)-1=V/Q/(Si-Se)=KB/Umax.V/Q/Si+1/Umax (2)
Form (2) may be useful to present for showing the graph plot that associates with the inversion of the substrate loading removal rate (V/Q/(Si - Se)) versus the total substrate loading rate (V/Q/Si). If the plot is linear, linear regression can be used to appraise the intercept and the slope. The outcome is a straight line portion of intercept 1/ Umax and a slope of KB/Umax. KB is suffusion constant (mg l-1min-1) and Umax is a constant for the maximum substrate removal rate (mg l-1min-1).
Results and Discussion
Determining best growth conditions of bacteria and fungi
Based upon PCR experiments, the purified fungi and bacillus were Aspergillus oryzae and Halobacillus dabanensis, respectively. The growth of Aspergillus oryzae and Halobacillus dabanensis was studied in Sabouraud-4% dextrose agar and BHI agar medium with different concentrations of NaCl of 5%, 10%, 15%, 20% and 25%. In the Sabouraud-4% dextrose agar and BHI agar medium, the best growth was obtained at NaCl concentration of 20%. For two microorganisms, the highest level of dry mass and the best growth were achieved at NaCl concentration of 20% in Sabouraud-2% dextrose broth and BHI broth medium. No more significant growth was observed in the medium at other NaCl concentrations. Aspergillus oryzae had the best growth at the temperature of 37 ºC which was procured after 72 h of single colonies with the brownish green pigments in the Sabouraud-4% dextrose agar medium. At temperature 28 ºC and 45 ºC, no growth was observed. However, Halobacillus dabanensis had the best growth at the temperature of 45 ºC released after 24-36 h of single colony.
Biofilm of Aspergillus oryzae and Halobacillus dabanensis
The optimum inoculation of Aspergillus oryzae into the saline wastewater and Sabouraud-2% dextrose broth medium for formation of biofilm were 15 ml and 8 ml, respectively. Optimum inoculation of Halobacillus dabanensis into BHI broth medium and saline wastewater for formation of biofilm were 5 Mcfarland to the amount of 3% and 8 Mcfarland to the amount of 5%. After 56 h, Aspergillus oryzae grew around the Ca-alginate bed, and after 30 h, the biofilm of Halobacillus dabanensis was organized. To ensure the formation of fungi and bacillus films, the samples of Ca-alginate granules were imaged by SEM (Fig.2 and Fig.3 ). On the other hand, as the Aspergillus oryzae and Halobacillus dabanensis were formed around Ca-alginate with optimum conditions in the saline wastewater, no significant thickness of Aspergillus oryzae and Halobacillus dabanensis was observed around Ca-alginate bed recorded by SEM images; therefore, it was disregarded set the Ca-alginate granules in the fixed bed column.
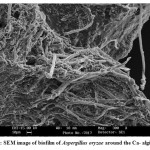 |
Figure 2: SEM image of biofilm of Aspergillus oryzae around the Ca- alginate. Click here to View figure |
 |
Figure 3: SEM image of biofilm of Halobacillus dabanensis around the Ca- alginate. Click here to View figure |
Effect of flow rates on removal of BOD5 and COD by biofilms of (Aspergillus oryzae and Halobacillus dabanensis) and, determination of kinetic constants
Biofilm is a conspicuous method upgrading the performance of bioreactors in removal of environmental pollution. In this research, the performance of bioreactors was analyzed taking into account the efficiencies of BOD5 and COD removal depending on the flow rates ranging between 2 to 6 ml min-1. According to the obtained results, when flow rate was increased from 2 to 6 ml min-1, the amounts of volumetric loading rate increased for both kinds of pollution. Tables 1 and 2 give the effect of flow rates on efficiency of BOD5 and COD removal by Aspergillus oryzae and Halobacillus dabanensis. An increase in the flow rate from 2 to 6 ml min-1 cased a decrease in removal of BOD5 and COD. This was due to the fact that in the high flow rates, the contact times of saline wastewater with biofilms as well as removal of BOD5 and COD content of saline wastewater in the column are decreased. The removal of BOD5 by Aspergillus oryzae and Halobacillus dabanensis decreased from 105 mg l-1 to 10 mg l-1, and from 105 mg l-1 to 20 mg l-1, respectively. Moreover, the removal of COD by Aspergillus oryzae and Halobacillus dabanens was decreased from 245 mg l-1 to 34 mg l-1 and from 245 mg l-1 to 45 mg l-1, respectively. These results show that Aspergillus oryzae and Halobacillus dabanensis can efficiently remove BOD5 and COD to the amounts of 90.4761% and, 86.1224%, 80.9523% and 81.6326%, respectively. Increased surface of biofilm in Aspergillus oryzae caused the maximum removal of BOD5 and COD, in comparison with Halobacillus dabanensis biofilm. This increased the volumetric mass transfer rate. Although biological treatment of saline wastewater is possible by using holophilic microorganisms; however, such microorganisms can tolerate salt to some extent. Accordingly, in contact with low salinity medium, after absorbing a large amount of salt, their tolerance will reduce and their cytoplasm will disintegrate and vice versa.
Table 1: Effect of hydraulic retention time on COD and BOD5 loading rate and on efficiencies of COD and BOD5 removal by Aspergillus oryzae.
|
HRT |
Q |
VLRCOD |
VLR BOD5 |
ECOD (%) |
E BOD5 (%) |
|
240 |
2 |
0.3333 |
0.1583 |
67.34 69 |
63.8095 |
|
720 |
2 |
0.0694 |
0.0375 |
79.5918 |
74.2857 |
|
1200 |
2 |
0.0283 |
0.0083 |
86.1224 |
90.4761 |
|
240 |
4 |
0.5 |
0.375 |
51.0204 |
14.2857 |
|
720 |
4 |
0.1138 |
0.0902 |
66.5306 |
38.0952 |
|
1080 |
4 |
0.0546 |
0.05277 |
75.9183 |
45.71 42 |
|
240 |
6 |
0.75 |
0.4166 |
26.5306 |
4.7619 |
|
480 |
6 |
0.3 |
0.1833 |
41.2244 |
16.1904 |
|
960 |
6 |
0.0937 |
0.07812 |
63.2653 |
28.5714 |
Omil et al. studied the treatment of saline wastewater from a sea food –processing industry with the salinity content similar to sea water and they could be able to achieve 70-90% removal of organic matter.25 Rovirosa et al. inspected the treatment of saline wastewater with Down-Flow Anaerobic Fixed Bed Reactor (DFAFBR). They found that at the HRT of 24h and salt concentrations range of 5 g l-1 to 15 g l-1, the reactor could reduce the COD concentration higher than 72%.28 Lefebvre et al. conducted an anaerobic digestion of tannery soak liquor characterized by high organic load and high salinity using Upflow Anaerobic Sludge Blanket reactor (UASB). They achieved a COD removal of 78% at a HRT of 5 days and a TDS concentration of 71gl_1.29 Kapdan and Erten studied the treatment of saline wastewater using up flow anaerobic packed bed reactor and Halanaerobium lacusrosei. They obtained a COD removal of 60% - 84% at the salt concentration of 3%.30
 |
|
 |
|
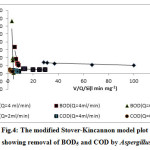
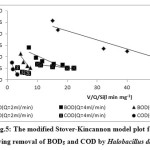
The modified Stover-kincannon model is widely used for analyzing experimental data from continuously operated systems, especially in continuously operated anaerobic systems.31, 32 The shore model is a commonly used mathematical model applied to determine kinetic constant values of immobilized systems.30 The model is used in thermophilic treatment systems, such as continuous anaerobic filter treatment systems for treating paper-pulp liquors,33 anaerobic hybrid reactors34 and anaerobic filter for soybean wastewater treatment.35 According, the continuously operated aerobic systems were used in this study. As the plots in Fig 4 and Fig 5 show, the data of the 2 ml min-1 flow rate are in more adherences with this model than the other flow rates. Maximum removal of BOD5 and COD was obtained at a HRT of 1200 min, after the Aspergillus oryzae and Halobacillus dabanensis films were remained in the column for 43 h and 24 h respectively. The highest removal of BOD and COD by Aspergillus oryzae was occurred after 1200 min. Due to the rapid growth of the Aspergillus oryzae, the biofilm was thickened, the column was reached the breakthrough point soon, and removal process was stopped. During the growth phase, the growth of Halobacillus dabanensis was stopped and interred to the death phase. Accordingly, the column reached to the breakthrough point.
According to the study based on the Stover model, the optimization conditions of the removal of COD efficiency constant of Umax and KB with the flow rate of 2 ml min-1 by Aspergillus oryzae and Halobacillus dabanensis, and the regression shown in Table 3.
Table 2: Effect of hydraulic retention time on COD and BOD5 loading rate and on efficiencies of COD and BOD5
removal by Halobacillus dabanensis.
|
HRT |
Q |
VLRCOD |
VLR BOD5 |
ECOD (%) |
E BOD5 (%) |
|
240 |
2 |
0.4166 |
0.275 |
59.1836 |
37.1428 |
|
720 |
2 |
0.0916 |
0.0430 |
73.4693 |
70.4761 |
|
1200 |
2 |
0.0375 |
0.0166 |
81.6326 |
80.9523 |
|
240 |
4 |
0.625 |
0.2875 |
38.7755 |
34.2857 |
|
720 |
4 |
0.1194 |
0.0597 |
64.8979 |
59.0476 |
|
1080 |
4 |
0.0648 |
0.025 |
71.4285 |
74.2857 |
|
240 |
6 |
0.8333 |
0.3166 |
18.3673 |
27.6190 |
|
480 |
6 |
0.3541 |
0.1291 |
30.6122 |
40.9523 |
|
960 |
6 |
0.1010 |
0.04479 |
60.4081 |
59.0476 |
Table 3: Comparison amount of Umax and KB and liner regression by Aspergillus oryzae and Halobacillus dabanensis
|
Aspergillus oryzae |
Halobacillus dabanensis |
|
|
Umax (mg COD l-1min-1) |
0.1449 |
0.1225 |
|
KB (mg COD l-1min-1) |
0.0003 |
0.00082 |
|
R2 (COD,Q 2ml min-1) |
0.9523 |
0.9345 |
|
R2 (BOD5, Q 2ml min-1) |
0.8932 |
0.8642 |
|
R2 (COD,Q 4ml min-1) |
0.8661 |
0.8819 |
|
R2 (BOD5, Q 4ml min-1) |
0.8404 |
0.7818 |
|
R2 (COD,Q 6ml min-1) |
0.7952 |
0.8562 |
|
R2 (BOD5, Q 6ml min-1) |
0.8625 |
0.8761 |
Conclusions
In the present study, the removal of BOD5 and COD was investigated by a fixed bed operation, using Aspergillus oryzae and Halobacillus dabanensis. The highest amounts of the BOD5 and COD removal were 90.4761% and 86.1224% by Aspergillus oryzae were achieved at the flow rate of 2 ml min-1since. The Aspergillus oryzae film has a better performance than the Halobacillus dabanensis film. The formation of Aspergillus oryzae film around Ca-alginate boosts shelf-life of the biofilm on the substrate by polysaccharide compounds on the surface of fungi cells and hydrogen bonds between the polysaccharide compounds and Ca-alginate which causes maximum adhesion of the fungus on the bed. This caused Aspergillus oryzae film to form regularly around Ca-alginate and provide maximum contact of the fungi with saline water. After 1200 minutes, the elimination process was stopped and the column reached the breakthrough point. The maximum substrate removal constant of 0.034 mg BOD5 l-1min-1 and the saturation constant of 0.00026 mg BOD5 l-1min-1 were calculated at the flow rate of 2 ml min-1 by Halobacillus dabanensis.
Acknowledgement
We express our deep gratitude to Mahmodieh Laboratory of Islamic Azad University- North Tehran Branch (IAU-NTB) for their final support. Special thanks for Genetic Engineering Central of Iran for their assistant in this project.
Reference
- Diaz, M. P., Boyd, K. G., Grigson, S. J. W. & Burgess J. G., Biodegradation of crude oil across a wide range of salinities by an extremely halotolerant bacterial consortium MPD-M, immobilized onto polypropylene fibers. Biotechnology and Bioengineering, 79(2): 145–153 (2002).
- Fakhru’l-Razi, A., Pendashteh, A. R., Luqman Chuah, A., Dayang Radiah, A. B., Madaeni, S. S. & Zurina Z. A., Review of technologies for oil and gas produced water treatment. Journal of Hazardous Materials, 170(2-3): 530–551 (2009).
- Campos, J. C., Borges, R. M. H., Oliveira Filho, A. M., Nobrega, R. & Sant’anna Jr, G. L., Oilfield wastewater treatment by combined microfiltration and biological processes. Water Research, 36(1): 95–104 (2002).
- Yogalakshmi, K. N. & Joseph, K., Effect of transient sodium chloride shock loads on the performance of submerged membrane bioreactor. Bioresource Technology., 101(18): 7054-7061 (2010).
- Pendashteh, A. R., Fakhru’l-Razi, A., Madaeni, S. S., Chuah Abdullah, L., Abidin, Z. Z. & Awang Biaka, D. R., Membrane foulants characterization in a membrane bioreactor (MBR) treating hypersaline oily wastewater. Chemical Engineering Journal, 168(1): 140-150 (2011).
- Uygur, A., Specific nutrient removal rates in saline wastewater treatment using sequencing batch reactor. Process Biochemistry, 41(1): 61–66 (2006).
- Taheri, E., Khiadani, M. H., Amin, M. M., Nikaeen, M. & Hassanzadeh, A. Treatment of saline wastewater by a sequencing batch reactor with emphasis on aerobic granule formation. Bioresource Technology, 111: 21-26 (2012).
- Abou-Elela, S. I., Kamel, M. M. & Fawzy, M. E., Biological treatment of saline wastewater using a salt-tolerant microorganism. Desalination, 250(1): 1–5 (2010).
- Kargi, F. & Dincer, A., Enhancement of biological treatment performance of saline wastewater by halophilic bacteria. Bioprocess Engineering, 15(1): 51–58 (1996).
- Ozalp, G., Gomec, Y. C., Gonuldinc, S., Ozturk, I. & Altinbas, M., Effect of high salinity on anaerobic treatment of low strength effluents. Water Science and Technology, 48(11-12): 207–212 (2003).
- Shi, K., Zhou, W., Zhao, H. & Zhang, Y. Performance of halophilic marine bacteria inocula on nutrient removal from hypersaline wastewater in an intermittently aerated biological filter. Bioresource Technology, 113: 280–287 (2012).
- Chowdhury, P., Viraraghavan, T. & Srinivasan, A., Biological treatment processes for fish processing wastewater – A review. Bioresource Technology, 101(2): 439–449 (2010).
- Zhao, X., Wang, Y., Ye, Z., Borthwick, A. & Ni, J. Oil field wastewater treatment in biological aerated filter by immobilized microorganisms. Process Biochemistry, 41(7): 1475–1483 (2006).
- Woolard, C. R. & Irvine, R. L., Treatment of hypersaline wastewater in the sequencing batch reactor. Water Research, 29(4): 1159–1168 (1995).
- Qin, L., Liu, Y. & Tay, J. H., Effect of settling time on aerobic granulation in sequencing batch reactor. Biochemical Engineering Journal, 21(1): 47– 52 (2004).
- Adav, S. S., Lee, D. J. & Tay, J. H., Extracellular polymeric substances and structural stability of aerobic granule. Water Research, 42(6-7): 1644– 1650 (2008).
- Wijffels, R. H. & Tramper, J., Nitrification by immobilized cells. Enzyme and Microbial Technology, 17(6): 482–492 (1995).
- Yan, J., Jetten, M. & Rang, J., Hu, Y. Comparison of the effects of different salts on aerobic ammonia oxidizers for treating ammonium-rich organic wastewater by free and sodium algina teimmobilized biomass system. Chemosphere, 81(5): 669–673 (2010).
- Reid, E., Liu, X. & Judd, S. J., Effect of high salinity on activated sludge characteristics and membrane permeability in an immersed membrane bioreactor. Journal of Membrane Science, 283(1-2): 164–171 (2006).
- Sun, C., Leiknes, T., Weitzenböck, J. & Thorstensen, B. Salinity effect on a biofilm- MBR process for shipboard wastewater treatment. Separation and Purification Technology, 72(3): 380–387 (2010).
- Sudarno, U., Bathe, S., winter, J. & Gallert, C., Nitrification in fixed-bed reactors treating saline wastewater. Applied Microbiology and Biotechnology, 85(6): 2017–2030 (2010).
- Woolard, C. R., & Irvine, R. L., Biological treatment of hypersaline wastewater by a biofilm of halophilic bacteria. Water Environment Research, 66(3): 230–235 (1994).
- Sakamoto, Y., Mashiko, T., Suzuki, A., Kawata, H. & Iwasaki, A. Development of encapsulation technology for synthetic seed. Acta Hortic, 319: 71-76 (1992).
- Murray, P. R., Baron, J., Jorgensen, J. H., Pfaller, M. A., Yolken, R. H., Manual of Clinical Microbiology, ASM press, Washington D.C, 358 (2003).
- Omil, F., Méndez, R. & Lema J. M. Anaerobic treatment of saline wastewaters under high sulphide and ammonia content. Bioresource Technology, 54(3): 269–278 (1995).
- Eaton, A. D., Clesceri, L. S., Rice, E. W., Greenberg, A. E., Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association, 2005.
- KosiÅ„ska, K. & MiÅ›kiewicz T., Performance of an anaerobic bioreactor with biomass recycling, continuously removing COD and sulphate from industrial wastes. Bioresource Technology, 100(1): 86–90 (2009).
- Rovirosa, N., Sánchez, E., Cruz, M., Veiga, M. C., Borja, R. Coliform concentration reduction and related performance evaluation of a down-flow anaerobic fixed bed reactor treating low-strength saline wastewater. Bioresource Technology, 94(2): 119–127 (2004).
- Lefebvre, O., Vasudevan, N., Torrijos, M., Thanasekaran, K. & Moletta, R. Anaerobic digestion of tannery soak liquor with an aerobic post treatment. Water Research, 40(7): 1492–1500 (2006).
- Kapdan, I. K. & Erten, B., Anaerobic treatment of saline wastewater by Halanaerobium lacusrosei. Process Biochemistry, 42(3): 449–453 (2007).
- Sandhya, S. & Swaminathan, K. Kinetic analysis of treatment of textile wastewater in hybrid column upflow anaerobic fixed bed reactor. Chemical Engineering Journal, 122(1-2): 87–92 (2006).
- Sponza, D. T. & Uluköy, A. Kinetic of carbonaceous substrate in an upflow anaerobic sludge blanket (UASB) reactor treating 2, 4 dichlorophenol (2,4 DCP). Journal of Environmental Management, 86(1): 121–131 (2008).
- Ahn, J. H. & Forster, C. F., A comparison of mesophilic and thermophilic anaerobic upflow filters treating paper-pulp-liquors. Process Biochemistry, 38(2): 256–261 (2002).
- Büyükkamaci, N. & Filibeli, A., Determination of kinetic constants of anaerobic hybrid reactor. Process Biochemistry, 38(1): 73–79 (2002).
- Yu, J., Wang, X. & Yue, P. L. Optimal decolorization and kinetic modeling of synthetic dyes by Pseudomonos strains. Water Research, 35(15): 3579–3586 (2001).







