Valorization of Some Untreated Low Cost Adsorbents for Water Pollution Control
Bashkim Thaçi1 , Majlinda Daci-Ajvazi1 * , Nexhat Daci2 and Salih Gashi2
DOI: http://dx.doi.org/10.12944/CWE.11.3.06
The present article describes the adsorption behavior of some low cost adsorbents such as olive waste, maize cobs, bentonitic clay, wheat bran, coal ash and coffee waste, with respect to Pb2+, Cu2+ and Zn2+ ions. The batch method was used and parameters such as electrical conductivity, pH, contact time, adsorbent dosage and initial and final concentration of metal ions were studied. All used adsorbents were effective, but coal ash was most effective, with total removal for all ions of over 90%, with highest percentage removal from 99.2% for Zn2+ ions and 97.5% of Pb2+. Maize cob was an effective adsorbent with maximal percentage removal of 92.6% for Zn2+ ions, wheat bran had highest removal of 93.7% for Pb2+ ions as did olive waste, 97% for Pb2+ ions. Coffe waste offers considerable promise as a low-cost natural adsorbent with highest efficiency in removal of Pb2+ with 97.5% while bentonitic clay having a structure with net negative charge, which is neutralized by positively charged species, resulted also as an effective low cost adsorbent with max total removal from 92.7% for Pb2+ to 80.9% for Zn2+ ions.
Copy the following to cite this article:
Thaçi B, Daci-Ajvazi M, Daci N, Gashi S. Valorization of Some Untreated Low Cost Adsorbents for Water Pollution Control. Curr World Environ 2016;11(3). DOI:http://dx.doi.org/10.12944/CWE.11.3.06
Copy the following to cite this URL:
Thaçi B, Daci-Ajvazi M, Daci N, Gashi S. Valorization of Some Untreated Low Cost Adsorbents for Water Pollution Control. Curr World Environ 2016;11(3). Available from: http://www.cwejournal.org/?p=16610
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2016-11-29 |
|---|---|
| Accepted: | 2016-12-21 |
Introduction
Through history, quality and quantity of water that was at human disposal, was adecisive factor for determining their welfare. At one time, clean fresh water supplies were considered inexhaustible. Only recently have we begun to understand that we will probably exhaust our usable water supplies and this can be directly attributed to human abuse in the form of pollution. Industrial activity alters the natural flow of materials and introduces novel chemicals into the environment which effluents contain toxic substances especially heavy metals, dyes, phenols, etc.,1 Some heavy metals are necessary in small amounts for normal development of biological cycles, however most of these heavy metals are becoming toxic at high concentration.2 Polluted water is aesthetically objectionable for drinking, irrigation, industrial activities and other purposes3 as pollutants alter physical, chemical and biological properties of water, hence affect human health and ultimately ecosystem. Different methods (adsorption, electrolytic or liquid extraction, electro dialysis, chemical precipitation, membrane filtration) have been developed for decontamination of industrial waters.4,5,6,7,8 From all methods used, adsorption has been found to be superior to other techniques for purification of water in flexibility and simplicity of design, ease of operation and insensitivity to toxic pollutants.9,10,11,12,13 When comparing the adsorption materials, one must have in mind cost as a very important parameter. However, an adsorbent can be assumed as low cost if it requires little processing, is abundant in nature, or is a byproduct or waste material from another industry. Some biosorbents can bind and collect a wide range of heavy metals with no specific priority, whereas others are specific for certain types of metals.14 Different low cost adsorbents have been used for wastewater treatment, some more effective than others.15,16,17 Activated carbon is usually derived from natural materials (biomass, lignite or coal) and has been a popular choice as an adsorbent for long time,18,19 but its high cost poses an economical problem. Different authors tried different low cost adsorbents like clays,20 microbial and plant derived biomass,21 chitin and zeolites,22 sawdust,23 rice husk,24 soybean hulls,25 sugarcane bagasse,26 etc.
In the last decade, olive oil production has increased by approximately 40 % worldwide and this implies a proportional increase in huge quantities of liquid and solid wastes. Double-fold advantage, with respect to environmental pollution, is to use such solid wastes and to convert them in inexpensive adsorbent for water pollution control. This way a part of solid waste material could be reduced, and the developed low-cost adsorbents can treat industrial wastewaters at a reasonable cost.27,28,29 Solid residues from corn production such as corn cobs can also be used as raw materials in the production of adsorbents.30,31 Wheat bran, another agricultural waste was studied for its adsorbent properties.32,33 In recent years there has been increasing interest in studying also natural waste materials that arise from food industry, e.g. coffee waste34,35 those that come through various industrial processes, like coal ash36,37 and other natural low cost materials, like bentonitic clays.38,39
In present study we’ve analyzed some low cost materials, olive waste, maize cobs, bentonitic clay, wheat bran, coal ash and coffee waste as potential adsorbents for removing of Pb2+, Cu2+ and Zn2+ ions from standard solutions.
Materials and Methods
Adsorbent
The starting materials, maize cobs, wheat bran, and coffee waste were obtained commercially from Kosovo, coal ash was obtained from Thermo Power Plants in Kosovo, bentonitic clay from Vitia, Kosovo while olive waste were obtained from olive oil industry in Ulqin, Montenegro. All the adsorbent used where sieved and dried at 105ºC to a constant weight.
In terms of physical-chemical properties, chemical composition of ash varies greatly, depending on the type of coal and its origin. Coal ash from our lignite type coal (Thermo power plants Kosova A and B) has specific inorganic composition with predominance of alkaline components (CaO and MgO), while bentonitic clay although also alumosilikate, has predominance of acidic constituents (SiO2). Adsorbents of agricultural origine have high mechanical strength, rigidity and porosity and they have polymeric groups like cellulose, hemi-cellulose, pectin, lignin and proteins as active centers for metal uptake.40
General procedure for adsorption studies
The sorption of Pb2+, Cu2+ and Zn2+ ions on used adsorbents (olive waste, maize cobs, bentonitic clay, wheat bran, coal ash and coffee waste) was studied using a batch technique. The general method used for this study is described as follows. The stock solution of PbSO4, CuSO4 and ZnSO4 at a concentration of 10 mg/L was used in all experimental runs. A known weight of adsorbent (1g and 5g) was equilibrated with Pb2+, Cu2+ and Zn2+ solutions of known concentrations in a stopped pyrex glass flask at a ï¬xed temperature in a thermostatic shaker bath (300 rpm) for a known period of time (30 min and 60 min). After equilibration, the suspension was filtered and analyzed with AAS.
Results and Discussion
Since heavy metals are natural components of the Earth's crust and they cannot be degraded or destroyed, their treatment is of special concern due to their recalcitrance and persistence in the environment. Manufacturing a diversity of adsorbents as raw materials is being studied (olive waste, maize cobs, bentonitic clay, wheat bran, coal ash and coffee waste) since these materials are renewable, usually available in large amounts and less expensive than other materials to. Use of waste materials as inexpensive adsorbents for removing of heavy metal ions from water and wastewater presents a great contribution in the reduction of costs for waste disposal, therefore contributing to environmental protection.
The sorption of Pb2+, Cu2+ and Zn2+ ions on used adsorbents was studied using a batch technique. Concentration of heavy metal ions before treatment was 4 mg/dm3 for Pb2+ ions, 3.57 mg/dm3 for Cu2+ ions and for Zn2+ ions it was 4.98 mg/dm3, respectively. pH of these untreated aqueous solutions were 5.29 for Pb2+ ions, 6.0 for Cu2+ ions and for Zn2+ ions pH was 5.4, respectively. Electrical conductivity for all heavy metal ions before treatment was 0.00 μS/cm. Results of treatment of each heavy metal aqueous solution with used adsorbents are given in Table 1.
From the results shown in Table 1 it can be noted that pH of aqueous solution of Pb2+, Cu2+ and Zn2+ ions after treatment with maize cobs, olive waste and wheat bran are almost the same as they were before treatment, while after treatment with coal ash pH are increased from8.3 to 11.3 due to predominance of alkaline components in our coal ash, while after treatment with bentonitic clay and coffee waste pH are slightly lower from 4.03 to 6.3, due to their acidic nature. Electrical conductivity of aqueous solution of Pb2+, Cu2+ and Zn2+ ions after treatment with all used adsorbents was slightly increased from 0.01 μS/cm to 5.15 μS/cm when 5g of adsorbents were used.
 |
|
 |
|
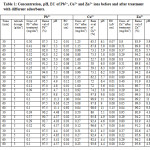
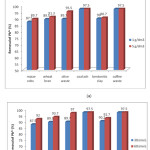
Fig.1: Precentage of removal of lead in dependence from (a) adsorbent dosage, 1g and 5g; and (b) from time, 30 min and 60 min.
Adsorbent dosage is an important parameter because this determines the capacity of an adsorbent for a given initial concentration of the adsorbate. The effect of adsorbent (olive waste, maize cobs, bentonitic clay, wheat bran, coal ash and coffee waste) dose on the adsorption of Pb2+ is presented in Figure 1a. As shown in this figure, results of percentage removal of Pb2+ ions are very high with all adsorbent used, ranging from 87.5% for maize cobs to 97.5% for coal ash and coffee waste. From this figure it can be noted that there is no significant impact in precentage removal of lead with the increase in adsorbent dosage from 1g to 5g. Slightly greater removal of lead is noticed with olive waste, 6.2%, while with maize cobs 2.2%, wheat bran 1.8% and bentonitic clay 0.8% the impact was very small. Coal ash and coffee waste demonstrated no effect when adsorbent dosage was increased.
Figure 1b shows the effect of contact time on percentage removal of Pb.2 It was observed that percentage removal slightly increases with contact time. Highest increase in percentage removal was observed with olive waste, 7.3% or bentonitic clay showed 2.5% increase in percentage removal with increase in contact time from 30 min to 60 min. Coal ash and coffee waste did not show any effect with increase of contact time.
 |
|
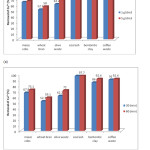
Figure 2(a) shows the effect of adsorbent dose (1 and 5 g/dm3 for 30 min) on the adsorption of Cu2+ ions. From these results it can be noted that coal ash was best adsorbent for removal of Cu2+ with 98.9% of removal while wheat bran removed 52.7%. Increasing adsorbent dose from 1g to 5g had more effect on removing of Cu2+ ions, e.g., adsorption on olive waste increased for 10.2% while with wheat bran increasing in percentage removal was 5.3%. Other adsorbents showed small increase for fivefold increase in adsorbent dosage, while coal ash showed no effect in percentage removal of Cu2+ ions.
The effect of contact time on percentage removal of Cu2 is shown in Figure 2(b). It was observed that increasing contact time from 30 min to 60 min had more effect on percentage removal of Cu2+ than it did with Pb2+. Highest increase in percentage removal was observed with olive waste, 10.2% and wheat bran 7.6%, while bentonitic clay and maize cob showed 6.4% and 5.3% respectively. Coal ash did not show any effect with increase of contact time.
 |
|
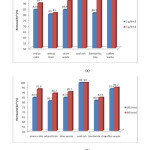
Figure 3(a) and 3(b) shows percentage removal of Zn2+ ions from all used adsorbents. Figure 3(a) shows results of percentage removal of Zn2+ after treatment with all adsorbent dosage of 1g and 5g for 30 min of contact time. It can be noted that adsorbent dosage had most effect in bentonitic clay, increasing percentage of removal for 13.3% with increasing of dosage for fivefold. Smaller impact was noted with olive waste 8.1%, maize cobs 5.8%, coffee waste 3%, wheat bran 1%, while coal ash showed no impact at all. Both graphs also confirms that most effective adsorbent on removal of Zn2+ was coal ash with over 99% of total removal and least effective was wheat bran with 80% of total removal.
Figure 3(b) shows that percentage of removal of Zn2+ ions in dependence of increasing contact time, from 30 min to 60 min, did not have any effect in coal ash, had a small effect in coffee waste 1.6%, 3% in bentonitic clay, 5.8% in olive waste, 7.9% in wheat bran and highest effect was noted for maize cob with 8.7% increase.
Conclusions
The present study shows that all adsorbents used were an effectual biosorbents for removal of Pb2+, Cu2+ and Zn2+ ions from aqueous solution. Maize cob was an effective adsorbent with maximal percentage of removal of 92.6% for Zn2+ and 66% for Cu2+ ions. Wheat bran was also effective adsorbent for highest removal of Pb2+ with 93.7% and lowest percentage of removal for Cu2+ with 52.7%. Another adsorbent that showed good sorption properties was olive waste, with highest removal from 97% for Pb2+ and lowest percentage removal for Cu2+ from 62%. Our preliminary results indicate that coffee waste offers considerable promise as a low-cost natural adsorbent. Highest efficiency was noticed in removal of Pb2+ with 97.5% while lowest percentage removal was noticed for Cu2+ with 91%. Bentonitic clay having a structure with net negative charge, which is neutralised by positively charged species, resulted also as an effective low cost adsorbent with max total removal from 92.7% for Pb2+ to 80.9% for Zn2+. Most effective adsorbent that we used was coal ash with total removal for all ions over 90%, with highest percentage removal from 99.2% for Zn2+ ions and 97.5% of Pb2+. For all adsorbent used adsorbent dosage and contact time did not have significant impact in their adsorption capacities. As conclusion all these agro and industrial waste materials, to overcome environmental pollution, are low cost, eco-friendly and easy alternative instead of using chemicals for the removal of heavy metals and other pollutants.
References
- Igwe, J.C., Abia, A.A., A bioseparation process for removing heavy metals from waste water using biosorbents. African J. Biotech., 5, 1167-1179, (2006).
- Kula, I., Ugurlu, M., Karaoglu, H., Celik, A., Adsorption of Cd(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. B Technol. 99, 492-501, (2008).
CrossRef - Velmurugan, P., Kumar, R. and Dhinakaran, V. G., Dye removal from aqueous solution using low cost sorbent. Int. J. Environ. Sci.,Vol. 1, pp. 7-14, (2011).
- Qdais, H.A., Moussa, H., Removal of heavy metals from wastewater by membrane processes: a comparative study. Desalination 163 (2) 105-110, (2004).
CrossRef - Gode, F. and Pehlivan, E. Adsorption of Cr(III) Ions by Turkish Brown Coals. Fuel Processing Technology, 86, 875-884, (2005).
CrossRef - Low, K.S., Lee, C.K., Ng, A.Y., Column study on the sorption of Cr(VI) using quaternized rice hulls. Technol. 68, 205-208, (1999).
CrossRef - Lacour, S., Bollinger, J. C., Serpaud, B., Chantron, P., Arcos, R., Removal of heavy metals in industrial wastewaters by ion-exchanger grafted textiles. Anal. Chim. Acta., 428 (1), 121–132, (2001).
CrossRef - Yu, L.Y., Shukla, S.S., Dorris, K.L., Shukla, A., Margrave, J.L., Adsorption of chromium from aqueous solutions by maple sawdust. Hazard Mater. B 100, 53-63, (2003).
CrossRef - Gupta, V.K., Ali, I., Advances in water treatment by adsorption technology, Nature protocols, vol 1, 2661-2667, (2006).
- Gupta, V.K., Ali, I., Water Treatment for Inorganic Pollutants by Adsorption Technology, Environmental Water, 29-91, (2013).
- Amit, B., Minocha, A.K., Conventional and nonconventional adsorbents for removal of pollutants. Indian Journal of Chemical Technology, 13, 203-217, (2006).
- Alinnor, I .J., Adsorption of heavy metal ions from aqueous solution by fly ash, Fuel, 86, pp. 853–857, (2007).
CrossRef - Jiuhui, Q.U., Research progress of novel adsorption processes in water purification: a review, Journal of environmental sciences, vol. 20, 1-13, (2008).
CrossRef - Volesky, B., Holan, Z.R., Biosorption of heavy metals. Biotechnol Prog 11:235–250, (1995).
CrossRef - Sanas, M., Gawande, S.M., Application of flyash-based filter bed in domestic wastewater treatment, International journal of innovative research in technology, 3(1), 83-85, 2016.
- Tripathi, A., Ranjan, M.R., Heavy Metal Removal from Wastewater Using Low Cost Adsorbents, J Bioremed Biodeg, 6, 315, (2015).
CrossRef - Ahsan, S., Kaneco, S., Ohta, K., Mizono, T., Kani, K., Use of some natural and waste materials for waste water treatment. Water Resorces. 35, 3738–3742, (2001).
CrossRef - Bestani, B., Benderdouche, N., Benstaali, B., Belhakem, M., Addou, A., Methylene blue and iodine adsorption onto an activated desert plant, Bioresource Technology, vol. 99, no. 17, pp. 8441–8444, (2008).
CrossRef - Girods, P., Dufour, A., Fierro, V., Rogaumea, Y., Rogaumea, C., Zoulaliana, A., Celzardc, A., Activated carbons prepared from wood particleboard wastes: Characterisation and phenol adsorption capacities, Journal of Hazardous Materials, 166, 491–501, (2009).
CrossRef - Cadena, F., Rizvi, R., Peters, R.W., Feasibility studies for the removal of heavy metals from solution using tailored bentonite. In Hazardous and industrial Wastes, Proceedings of the Twenty – Second Mid-Atlantic Industrial Waste Conference, Drexel University, pp. 77-94, (1990).
- Sarabjeet, S.A.,Dinesh, G., Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresource Technology, 98, 2243–2257, (2007).
CrossRef - Moattar, F., Hayeripour, S., Application of Chitin and Zeolite adsorbents for treatment of low level radioactive liquid wastes. International Journal of Environmental Science & Technology, Vol. 1, No. 1, pp. 45- 50, (2004).
CrossRef - Bryant, P.S., Petersen, J.N., Lee, J.M., Brouns, T.M., Sorption of heavy metals by untreated red sawdust, Applied Biochemistry and Biotechnology, vol. 34-35, no. 1, pp. 777–78, (1992).
CrossRef - Ajmal, M., Rao, R.A.K., Anwar, S., Ahmad, J., Ahmad, R., Adsorption studies on rice husk: removal and recovery of Cd (II) from wastewater. Technol., 86, 147–149, (2003).
CrossRef - Marshall, W.E., Wartelle, L.H., Boler, D.E., Johns, M.M., Toles, C.A., Enhanced metal adsorption by soybean hulls modified with citric acid. Technol., 69, 263–268, (1999).
CrossRef - Ayub, S., Ali, S.I., Khan, N.A., Study on the removal of Cr(VI) by sugarcane bagasse from wastewater. Res. J., 2 (2), 233–237, (2001).
- Calero de Hoces M., Blázquez García,. G., Gálvez, A.R., Martín-Lara, M.A., Effect of the acid treatment of olive stone on the biosorption of lead in a packed-bed column. Ind Eng Chem Res 49:12587–12595, (2010).
CrossRef - Aziz, A., Elandaloussi, E.H., Belhalfaoui, B., Ouali, M.S., De Ménorval, L.C., Efficiency of succinylated-olive stone biosorbent on the removal of cadmium ions from aqueous solutions. Colloids Surf B Biointerfaces 73:192–198, (2009).
CrossRef - Babakhouya, N., Aksas, H., Boughrara, S., Louhab, K., Adsorption of Cd(II) Ions from Aqueous Solution using Mixed Sorbents Prepared from Olive Stone and Date Pit. Journal of Applied Sciences, 10: 2316-2321, (2010).
CrossRef - Arunkumar, C., Perumal, R., Lakshmi-Narayanan, S., Arunkumar, J., Use of Corn Cob as Low Cost Adsorbent for the Removal of Nickel (II) From Aqueous Solution. International Journal of Advanced Biotechnology and Research, Vol 5, 3, 325-330, (2014).
- Tsai, W.T., Chang, C.Y., Wang, S.Y., Chang, C.F., Chien, S.F., Sun, H.F., Cleaner production of carbon adsorbents by utilizing agricultural waste corn cob. Res. Conserv. Recycl, 32, 43-53, (2001).
CrossRef - Bulut, Y., Baysal, Z., Removal of Pb(II) from wastewater using wheat bran. Journal of Environmental Management 78, 107–113, (2006).
CrossRef - Ozer, A., Özer, D. and Özer, A., The adsorption of copper (II) ions onto dehydrated wheat bran (DWB): determination of the equilibrium and thermodynamic parameters, Process Biochemistry, Vol. 39, pp. 2183-2191, (2004).
CrossRef - Kyzas, G.Z., Commercial Coffee Wastes as Materials for Adsorption of Heavy Metals from Aqueous Solutions. Materials, 5(10), 1826-1840, (2012).
CrossRef - Djati Utomo, H., Hunter, K.A., Adsorption of heavy metals by exhausted coffee grounds as a potential treatment method for waste waters. e-J. Sci. Nanotech. Vol. 4 (2006) 504-506, (2006).
- Alinnor, I .J., Adsorption of heavy metal ions from aqueous solution by fly ash, Fuel, 86, pp. 853–857, (2007).
CrossRef - Kirk, D.W., Charles, Q., Jia, J.Y., Alan, L.T., Wastewater remediation using coal ash, Journal of Material Cycles and Waste Managment, 5, 5–8, (2003).
CrossRef - Kubilay, Åž., Gürkan, R., Savran, A., Åžahan, T., Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption, 13; 41-51, (2007).
CrossRef - Vega, J.L. Ayala, J., Loredo, J., Iglesias, J.G., Bentonite as adsorbent of heavy metal ions from mine waste leachates, 9th International Mine waste Congress, (2005).
- Anwar, J., Shafique, U., Waheed-uz-Zaman, Salman, M., Dar, A., Anwar, S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour Technol. 101(6):1752-5, (2010).
CrossRef







