Study of Adsorption Phenomena by Using Almond Husk for Removal of Aqueous Dyes
M. Bhanuprakash1 * and S. L. Belagali1
DOI: http://dx.doi.org/10.12944/CWE.12.1.10
Among air and soil, water pollution is considered as an important one. Deterioration of water resources by the addition of various pollutants leads to the major threat to water quality and use of water for domestic purpose which leads to unaesthetic. For the plants, animals and human beings dyes are considered as most hazardous among various water pollutants. The present paper describes the adsorption behaviour of adsorbent almond husk with respect to dyes of Crystal violet, Bromocresol green, Pararosaniline and Victoria blue was investigated. The batch method was used and parameters like pH, adsorbent dosage, contact time and initial and final concentration of dyes were studied. Adsorbent used to be effective, with total removal of all dyes of 90%, with higher percentage removal from bromocresol green 97.5%, Crystal violet 96.9%, Pararosaniline 95.6% and Victoria blue 95%. Almond husk was an effective adsorbent with maximum percentage removal of 97.5% bromocresol green. Adsorbent was analysed by the instrument of scanning electron microscopy and Fourier infrared spectroscopy. In the present study almond husk almond husk was studied as a very good adsorbent for the removal of dyes from the aqueous media. Isotherm model of Langmuir, Freundlich and Dubinin-Radushkevich were considered to be favourable.
Copy the following to cite this article:
Bhanuprakash M, Belagali S. L. Study of Adsorption Phenomena by Using Almond Husk for Removal of Aqueous Dyes. Curr World Environ 2017;12(1). DOI:http://dx.doi.org/10.12944/CWE.12.1.10
Copy the following to cite this URL:
Bhanuprakash M, Belagali S. L. Study of Adsorption Phenomena by Using Almond Husk for Removal of Aqueous Dyes. Curr World Environ 2017;12(1). . Curr World Environ 2017;12(1). Available from: http://www.cwejournal.org/?p=16796
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2017-01-11 |
|---|---|
| Accepted: | 2017-02-24 |
Introduction
Waste water from industries represents a challenge to biological and conventional physicochemical treatment methods considering for both effluent composition and volume of discharged.1 Among various industries, textile industries waste water discharge is a major environmental problem throughout the world, because it contains various synthetic dyes which lead to environmental pollution.2 It has been considered that using of more than 10,000 (worldwide) commercial dyes by textile and manufacturing industries, consumption of dyes 1000 tons/year. During dyeing process 10 to 15% of these dyes is discharged as effluents in to the waste streams.3 Industries like paper, leather tanning, cosmetic, food, woollen and carpet also contributes the pollution load.4 Most of the dyes released by textile industries have complex aromatic structure, resistant to light and harmful to the aquatic environment, biological activity and another degradative process.5 Some of the dyes are carcinogenic which leads to skin irritating, allergic dermatitis, dysfunction of kidney, liver, brain and central nervous system.6,7 Decolorization of textile waste water is the worldwide problem. Many treatment methods have been applied for the removal of textile dyes from wastewaters which include adsorption, nano-filtration, electro kinetic coagulation, ozonisation and precipitation, advanced chemical oxidation, electrochemical oxidation, supported liquid membrane, liquid-liquid extraction and biological process ,8 coagulation,9 Ozonisation and membrane separation,10 anaerobic decolourisation,11 Oxidation,12 flocculation13 and adsorption.14 Adsorption has evolved as one of the most effective physical processes for removal of dyes. The number of adsorbents like Ficus racemosa plant barks,15 Water hyacinth,16 Silica gel,17 Hazelnut shells,18 Sugarcane bagasse,19 Coralwood tree legume pod,20 Aspergillus tamarii,21 Rice Husk,22 agriculture residue based adsorbent,23 Various adsorbent,24 Biomass ,25 Composite adsorbent,26 Low cost adsorbents,27 Potatohusk.28 The literature survey reveals that almond husk has not been used as an adsorbent for the synthetic dyes such as Crystal violet, Victoria blue, bromocresol green and pararosaniline. So, we considered this material for further observation.
Materials and Methods
Preparation of adsorbent for study
From bakery and sweet shops, almond husk was collected. It was washed with water, dried in sunlight followed by hot air oven at 1000 c. The adsorbent was made into small pieces by hand and then it was crushed into powdered by mixer grinder at 5 to 10min. It was sieved by 2mm mesh to get uniform particle size for adsorption. The adsorbent was treated by 2N NaOH and 2N HCl followed by washed from distilling water to maintain pH neutral condition.
Preparation of dye solutions
100 ppm of (Crystal violet, Victoria blue, Bromocresol green and Pararosaniline) different dye solutions were prepared by dissolving 10mg of dye in 100ml of distilling water and was diluted to required concentrations. For all dye preparation procedure was same. We used simple possible equipment one can have in the lab for the analysis of the dye and the adsorbent sample that is UV-visible spectrophotometer and pH instruments were used. Scanning electron microscopy and Fourier transform infrared spectroscopy instruments was used for the surface analysis to confirm adsorption.
Experimental section
Batch Experiment
The 100ml stock solution was taken to conduct batch mode experiments for dye adsorption. The parameter of pH, adsorbent dose, concentration of adsorbate and contact time was studied. For the process of adsorption of the dye solution adsorbent was kept aside for adsorption to take place.
10ppm/L dye solutions were taken in five number of 25ml beakers for that adsorbent of 0.25g to 1.25g was added and adsorption was studied.
25ml samples with different concentrations of dye solution (2, 4, 6, 8 and 10 ppm) were taken in five beakers with a constant dose of 0.25g adsorbent added to each and allowed the process to go at room temperature.
25ml samples of (2 to 10ppm) of dye solutions taken in five beakers and adsorbent of varying doses of 0.25g to 1.25g put in an increasing manner. The solution was placed aside for adsorption. For the effect of contact time, a 25ml sample of 10ppm dye solution was studied by adding a 0.25g dose of adsorbent.
Adsorption isotherms
In order to determine the sorption potential of adsorbent, the study of sorption isotherm was essential in selecting an adsorbent for the removal of the dyes. The adsorption process was studied with the Freundlich and Langmuir isotherms (Adamson, 1960).29
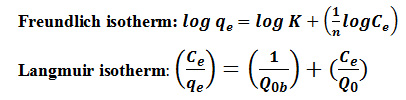
where, qe is the amount of dye adsorbed per unit mass of adsorbent at equilibrium (in mg/g), K and n are respectively the measures of sorption capacity and intensity of adsorption, Ce is the equilibrium concentration of dyes (in mg/L) and Qo and b are the Langmuir constants indicating the sorption capacity (in mg/g) and energy of adsorption (in gm/L) respectively from the slope and intercept.
Kinetics of the Adsorption
The adsorption kinetics were found to be of the first order. The following equation proposed by Kannan and Vanamudi (1991)30 was employed for adsorption data:
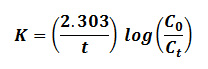
where, Co and Ct are concentrations of dyes at zero time and at time t (min). The values of log (Co/Ct) were found to be linearly correlated with the contact time for all the above dyes.Further, the essential characteristics of the Langmuir isotherm can be described in terms of a dimensionless constant, namely a separation factor or equilibrium parameter, RL which was defined by Weber and Chakravorti (1974) [31] in the equation: RL= l/(labia), where, b is the Langmuir constant and ci is the initial concentration of dye (in ppm). The value of the parameter, RL indicates the nature of the isotherm as given below. A standard RL value which indicates the nature of the developed Model shows in the table 1.
Table 1: Nature of the isotherm and their range
|
RL value |
Nature of isotherm |
|
RL > 1 0 < RL < 1 RL =1 RL = 0 |
Unfavorable Linear Favorable Irreversible |
The Dubinin-Radhuskevich isotherm model is another empirical model which initially formulated for the adsorption process following a pore filling mechanism (Dubinin 1960).32 It is generally applied to express the adsorption process occurred onto both homogeneous and heterogeneous. The non-linear expression of dubinin-radushkevich isotherm model can be illustrated as
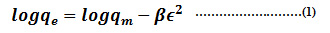
Where qe = amount of adsorbate in the adsorbent at equilibrium (mg/g); qm = theoretical isotherm saturation capacity mg/g; β = Dubinin-Radhuskevich isotherm constant (mol2/kg2) and Æ = Dubinin-Radhuskevich isotherm constant.
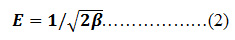
β can be denoted as isotherm constant mean while the parameter Æ can be calculated as
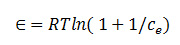
Where R,T and Ce represent the gas constant (8.314 J/mol K), absolute temperature (K) and adsorbate equilibrium concentration (mg/l).
Results and Discussion
The rate of adsorption of an adsorbent for an adsorption varies with different conditions i.e adsorbate concentration, adsorbent dose, pH and contact time, etc. In the present study, the effect of adsorption on different condition was carried out to determine the adsorption rate.
Effect of pH on the rate of adsorption
The table 2. shows the time taken for the adsorption of different dyes at acidic and neutral media.
Table 2: Adsorption of dyes with different pH
|
Dyes used |
Adsorption time at different pH |
||
|
Acidic (hrs) |
Neutral (hrs.) |
Basic (hrs.) |
|
|
Crystal violet |
LA |
5 |
LA |
|
Bromocresol green |
7 |
LA |
LA |
|
Pararosaniline |
6 |
LA |
LA |
|
Victoria blue |
5 |
LA |
LA |
Note: LA-less adsorption
From the data: Adsorption was carried out in neutral media for Crystal violet, whereas Bromo cresol green, pararosaniline and victoria blue were in the acidic media.
Effect of adsorbent dose
0.25g to 1.25g of the adsorbent dose was added to five numbers of 25ml samples of 1% dye for adsorption study. Adsorption was increased by increasing of dose for all dye solution (Fig. 1).
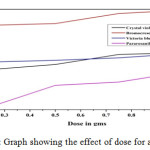 |
|
Effect of concentration
25ml samples of (2-5%) concentration of dye solution taken in five beakers at room temperature for that constant dose 0.25g added separately was studied for adsorption, which indicates that the concentration of the dye solution increases, the adsorption of the adsorbent decreases. (Fig. 2). Adsorption rate was decreased due to increase in concentration for all dye solution.
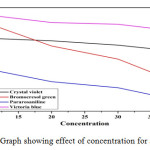 |
|
Effect of Dose v/s Concentration
The varying adsorbent dose of (0.25g to 1.25g) was added in series to five numbers of 25ml samples of (2 to 5%) dye solution and it was kept for adsorption to take place. From the result which indicates, concentration of the dye solution and dose are correlated with each other (Fig.3)
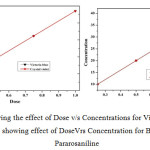 |
|
Effect of contact time
25ml of 10ppm dye solution added with 0.25g of adsorbent was investigated. From the result of all the dyes as the contact period increases adsorption increases. The contact time of all dye solution was within 12hrs.
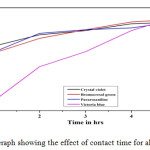 |
|
Scanning Electron Microscopic Image
The scanning electron microscope graph was taken at 50µm resolution. In the fig.a unadsorbed material shows voids and holes like structure with the irregular, rough surface area which are considered as active sites, for the adsorption of dyes.
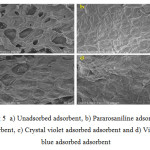 |
|
While on careful observation the adsorption was not same for all the dyes. In fig.5 (b ) while treating with Pararosaniline dye, all voids are filled and it looked to be covering all the surface area. Similarly, in the case of Crystal violet dye fig.5(c), there is a layer of formation on the active surface layer. But not completed as in the case of Pararosaniline, this indicates that the adsorbent can be further used for adsorption of Crystal violet dye. While looking at the material adsorbed with Victoria blue dye fig.5 (d) the active voids can be seen in unadsorbed material, but during the adsorption in looks to be a layer formation on voids covering the whole surfaces. This looks to be saturated when considering the other two results. It seems that the material has uptake the highest possible amount of dye,that is further adsorption leads to the rupture of the layer and affect the adsorption process.
Fourier transforms infrared spectroscopy instrumental analysis is carried out to the adsorbent dyes of Crystal violet, Pararosaniline and Victoria blue. The FT-IR spectra show in fig 6.
 |
|
The adsorption studies were also studied by the FT-IR spectral analysis the adsorbent also has some of the functional groups and also present in the dyes we can comprise both the unadsorbed and adsorbed adsorbent and can correlate the results. Because some of the interaction may be present in the functional groups of the dye and adsorbent. After adsorption, it can lead to the masking of some functional groups in the adsorbent, it may due to the chemical interactions between them. An adsorbent with crystal violet shows the absence of the peaks at 1424 cm-1 which were present in the unadsorbed samples and the intensity of other peaks decreased. Pararosaniline adsorbed adsorbent shows the strong and wide peaks in at 3200-3300 which indicates the amine group which is present in the dye and also shows the absence of the peaks at 1424cm-1 and also observe that increased Intensity of aromatic C-H stretching vibrations, because of the Victoria blue has plenty of aromatic C-H groups. From the above study, we can conclude that the distribution of functional groups differs in unadsorbed and adsorbed material, there we observe that disappearance of stretching frequency peak at 1424 cm-1 (Nitro group) and increased intensity in the peaks of aromatic C-H and NH2 groups. This change in functional group distribution in IR spectroscopy indicates the adsorption has taken place.
Adsorption Isotherm
Adsorption isotherm of Langmuir, Freundlich, and D-R isotherm is carried out on dyes of Bromocresol green, Crystal violet, Pararosaniline and Victoria blue. Looking at the results of adsorbent with different dyes, it is following the Fredlich Isotherm. It can be noted that the solpes with different dyes are above 2 and the interception of adsorbent with crystal violet dye is 3.03, indicating the effectiveness of adsorbent. The other isotherms are also displayed in the Table 3.
Table 3: Different adsorption isotherm studies
|
Adsorption Isotherm |
Bromocresol green |
Crystal violet |
Pararosaniline |
Victoria blue |
|
Langmuir: Slope(a) Intercept (b) Correlation Coefficient |
1 0.06 1.08 |
0.14 0.002 0.152 |
1.3 1.19 1.04 |
1.15 0.533 0.993 |
|
Freundlich: Slope(a) Intercept (b) Correlation Coefficient |
2.72 1.51 0.2 |
2.3 3.03 0.35 |
2.0 1.53 1.0 |
1.64 1.16 0.53 |
|
Kinetics of adsorption: 102k/min. Correlation Coefficient |
7.67x10-03 0.2 |
9.7x10-03 0.35 |
0.010 1.0 |
0.022 0.53 |
|
D-R isotherm: Slope (β) Intercept (qm ) D-R value |
0.015 0.045 5.88 |
2.23x10-07 5.9x10-06 4.74x10-07 |
0.72x104 0.21x104 8.33x10-3 |
0.86x109 0.5x108 2.41x10-10 |
Conclusion
Almond husk has shown excellent result as an adsorbent of dyes. To come to this conclusion adsorbent was exposed to various test with respect to concentration of dyes, pH, contact time and dosage of adsorbent was also analyzed. Based on these various isotherms were defined which showed that adsorbent has the ability to remove dyes from the aqueous system.
Acknowledgment
One of the Authors, Mr.Bhanuprakash M, is grateful to UGC Non-NET fellowship scheme, and thankful for the instrumentation facility to institute of excellence of the University of Mysore.
References
- Arivoli S, Nandhakumar V,Thiruchelvi M, Ramuthai S and Vijayakumaran V, Rhodamine B adsorption-kinetic, mechanistic and thermodynamic studies, E-Journal of Chemistry,6(SI), (S363-S373) 2009.
- Vandevivere C, Bianchi R and Verstraete W, “Treatment and reuse of wastewater from the textile wet- processing industry: Review of emerging technologies,” J. Chem. Technol. Biotechnol., 72, (289) 1998.
- Reisch M S. Asian textile dye makers are a growing power in changing market, Chemical & Engineering News., 74: (10-12),(1996).
CrossRef - Nigam P, Armour G, Banat I M, Singh D and Marchant R, Physical removal of textile dyes and solid state fermentation of dye-adsorbed agriculture residues, Bioresour Technol, 72,(219-26)2000.
CrossRef - Joshi M, Bansal and Purwar R , Colour removal from textile effluents, Indian J. of Fibre and Text. Res., 29, (239-259), 2004.
- Onyechi, C.A., Textile Wastewater Treatment Using Activated Carbon from Agro Wastes. M. Eng. Thesis, De-partment of Chemical Engineering, Nnamdi Azikiwe University, Awka (2014).
- Salleh M.A.M., Mahmoud D.K, Karim W.A.W.A. and Idris A., Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: Comprehensive Review. Desalination, 280, (1-13), (2011).
CrossRef - Mahmoud,A.S, Ghaly A.E. and Brooks M.S, Removal of Dye from Textile Wastewater Using Plant Oils un-der Different pH and Temperature Conditions, American Journal of Environmental Science, 3, (205-218),(2007).
CrossRef - Niranjan P. and Karthikeyan J, Colour removal from dye effluents by chemical coagulation - Basic and Disperse dyes, Indian J. Environ. Prot., 12, (599-603) 1992.
- Churchley J.H., Removal of dye house color from sewage effluent The use of a full-scale ozone plant, Water Sci.Tech., 30, (275-284) 1994.
- A. Removing acid dyes from textile wastewater using biomass for decolouration’ Am.Dyet.Rep., 83,(1721) 1994.
- Mittal A.K. and Venkobachar C, Uptake of cationic Dyes by Sulfonated Coal: Sorption Ind.Eng.Chem. Res. 35(4),(1472-1474)1996.
CrossRef - Neelamegani R., Baskaran V., Dhancekar R and Viruthgiri T., Decolourization of synthetic dyes using rice straw attached Pleurotus ostreatus, Indian J. Chem, Techno., 11, (622-625) 2004.
- McKay G, Ramprasad G and Pratap mowli P, Equilibrium studies for the adsorption of dyestuffs from aqueous solutions by low-cost materials, Air Soil Poll., 29, (274-283) 1986.
- Sujitha R and Rabindranath K, Removal of Coomassie brilliant blue dye from waste waters using active carbon derived from barks of Ficus racemosa plant, Der Pharmacia Lettre, 8 (10):7(2-83) 2016.
- Murali k and Uman R.N, Removal of a basic dye( methylene blue ) Using low-cost absorbent: water hyacinth., Int J Adv Engg Tech, 7(2),(386-391) 2016.
- Gaikwad R.W and Misal S.A, Sorption Studies of Methylene Blue on Silica Gel, international Journal of Chemical Engineering and Applications, 1(4)
- Fathi M. R. Asfaram A, Investigation of Kinetics and Equilibrium Isotherms of Direct Red 12B Dye Adsorption on Hazelnut Shells, Journal of Chemical Health Risks, 1(2): (1-12), 2011.
- Sachin M.Kanawade and R.W.Gaikwad, Removal of Dyes from Dye Effluent by Using Sugarcane Bagasse Ash as an Adsorbent, International Journal of Chemical Engineering and Applications, 2( 3),
- Bhanuprakash M, BelagaliL and Geeta K.S, Removal of dye from aqueous solution using coral wood tree legume as a natural adsorbent, The Ecoscan, 8(1&2), (123-126) 2015.
- El-Batal A.I, Hashem A.M, Hassan M.S and Helal A.H, Removal of dyes from textile wastewater using treated Aspergillus tamarii biomass in batch and column reactor, world applied sciences journal 19(9): (1305-1310) 2010.
- Shafiqul Alam A.M , Mohammad Arifur Rahman and Ruhul Amin S.M , Removal of Methylene Blue from Waste Water Using Activated Carbon Prepared from Rice Husk, Dhaka Univ. J. Sci. 60(2): (185-189) 2012.
- Kharat D S., Preparing agricultural residue based adsorbents for removal of dyes from effluents-a review, J.Chem.Eng., 32(1) 2015.
CrossRef - Murugan T., Ganapati A. and Valliappan R., Removal of Dyes from Aqueous Solution by Adsorption on Biomass of Mango (Mangifera Indica) Leaves, E-Journal of Chemistry, 7(3), (669-676) 2010.
CrossRef - Madhura Chincholi, Priyanka Sagwekar,Charmi Nagaria ,Sunil Kulkarni and Sonali Dhokpande, Removal of dye by adsorption on various adsorbents: a review, International journal of science, engineering and tecnological research,3(4)
- Jayanta Kumar Basu, Monal Dutta, Md Hassan Faraz, Nitin Gautam and Adarsh Kumar. Fixed-bed Column Study of Textile Dye Direct Blue 86 by using A Composite Adsorbent, Archives of Applied Science Research, 4 (2):(882-891) 2012.
- Ramakrishnaiah C.R and Arpitha D.N, Removal of Colour from Textile Effluent by Adsorption Using Low-Cost Adsorbents, International Rese. J. Pure Chem, 4(5):(568-577) 2014.
CrossRef - Pooja and Shrivastava, Study on the color removal of basic dye by potato husk as an Adsorbent, Chem. Biologl. Phys. Science. 2(2), (597-600). 2012.
- Adamson A. W. Physical chemistry of surfaces. Interscience Publ. Inc., 56(116) 1960.
- Kannan N. and Vanamudi A study on the removal of chromium (VI) by adsorption on lignite coal. Indian J. Env. Prot.11(4): (241-245) 1991.
- Weber T.W. and Chakravorti. Pore and solid diffusion models for fixed bed J. Am. Inst. Chem. Eng. 2: (228-238) 1974.
CrossRef - Dubinin M. M, The potential theory of adsorption of gases and vapors for adsorbent with energetically non-uniform surface, Chem. Rev., 60, (235-266) 1960.
CrossRef







