Utilization of a Material Derived from Animal Waste in the Biosorption of Pb(II) Ions – Isothermal/ Thermodynamic, Kinetics and Statistical Studies
J. Anuradha1 , N. Muthulakshmi Andal1 * and N.S. Gayathri1
DOI: http://dx.doi.org/10.12944/CWE.12.2.29
Heavy metal contaminations via industrial wastewaters endure as startle pollutants due to their nondestructive nature, toxicity, bioamplification and bioaccumulation. Removal of Pb(II) from aqueous solutions using animal waste is presented in this study. Acid treatment of the collected animal waste is done, further subjected to FTIR, SEM / EDAX analysis to study the morphology and presence of surface functional groups. Prefatory batch studies are performed to experiment the effects of sorbent particle size / dosage, contact time, initial metal ion concentrations and pH of the medium. The studies reveal excellent chelating ability of the treated material with 99.9% Pb(II) removal at a pH 5, the calculated adsorption capacity being 62.32 mg/g. Desorption/ Regeneration studies are carried out to assess the quantitative aspect of the metal laden material. Isothermal verification, thermodynamic parameters and kinetics of adsorption were applied. A comparison of isothermal models viz., Langmuir, Freundlich, Tempkin and DKR reveal the fit in of linearity to be best suited for Langmuir plot. Thermodynamic studies imply the process to be favorable, exothermic and spontaneous in nature. The sorption kinetics exhibits the system to be simulated well by pseudo-second-order kinetic model. A scientific basis for monitoring the Pb(II) removal is done with statistical data verification using descriptive and ANOVA tools. The results promote the employment of chosen animal waste material as an excellent biosorbent, in trapping toxic metal ions.
Copy the following to cite this article:
Anuradha J, Andal N. M, Gayathri N. S. Utilization of a Material derived from Animal Waste in the Biosorption of Pb(II) Ions – Isothermal/ Thermodynamic, Kinetics and Statistical Studies. Curr World Environ 2017;12(2). DOI:http://dx.doi.org/10.12944/CWE.12.2.29
Copy the following to cite this URL:
Anuradha J, Andal N. M, Gayathri N. S. Utilization of a Material derived from Animal Waste in the Biosorption of Pb(II) Ions – Isothermal/ Thermodynamic, Kinetics and Statistical Studies. Curr World Environ 2017;12(2). Available from: http://www.cwejournal.org?p=1007/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2017-03-03 |
|---|---|
| Accepted: | 2017-05-01 |
Introduction
Release of large quantities of pollutants into the natural environment has resulted in a number of environmental problems. Heavy metals discharge is of more concern due to its toxicity and tendency of bioaccumulation.1 The anthropogenic sources of metals include industrial, petroleum contamination, sewage disposal and many a lot.
Lead is the most recycled non-ferrous metal, utilized in the manufacturing, construction and chemical industries due to its malleable and ductile properties. Its primary uses involves, the manufacturing of batteries, petrol additives, rolled and extruded products, alloys, pigments, cable sheathing, shot and ammunition.2 Lead is toxic to multiple organ systems, even at low concentration.
The nervous system of the fetus and infant is especially susceptible to lead, which can cross the placenta and penetrate the blood-brain barrier. The consequences are loss of intelligence, growth and disruption of behavior. Recent researches indicate that lead can damage the infant brain at blood levels 5 µg/dL itself.3
Heavy metal removal techniques include chemical precipitation, chemical oxidation or reduction, evaporation, ion exchange and membrane technologies. Biosorption, an economic- effective and simple method, proceed via., physic-chemical interaction between metal ions and the functional groups present on the adsorbents’ surface. Utilization of commercial expensive adsorbents is now getting replaced by low cost materials, owing to its availability in large quantities. Once, if the solid material could be used as a sorbent, it provides another advantage of solid waste disposal.4
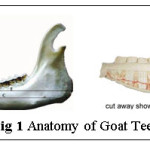
Bones are a part of the composite that gives shape and support to animals. Tooth enamel and bone have a quite dissimilar composition and therefore have different physical and mechanical properties. Tooth enamel is the hardest and most highly mineralized substance in the body. It is 96% mineral (calcium is found as Hydroxyapatite (Ca10[PO4]6[OH]2), with water and 4% protein.5 The high mineral contents provide it strength and hardness, but also brittleness. Goat teeth routed in jaw are a solid waste dumped in butcher’s shop, later being discarded as a no cost waste. It is a shear litter other than being helpful in determining the age of a goat. The anatomical image of goat teeth is shown fig 1.
 |
|
The waste goat teeth, is employed in the current study for the removal of Pb(II) ions. The choice of the material is based on the fact that replacement of calcium ions from the mineral calcium phosphate by Pb(II) ions is favorable,6 arranged in a crystalline structure known as Hydroxyapatite. The adsorption of Pb(II) on goat teeth can be attributed to many factors such as low solubility, complex physical form, relatively high content of reactive groups that can serve as exchange sites.
Biosorption efficiency depends upon many factors viz., sorbent dosage/ particle size, contact time, temperature and metal ion concentration/ pH. The equilibrium data for an evaluation of the ability of isothermal and kinetic models to describe the process behavior is calculated. Seldom studies have been reported elsewhere in the literature, on the optimization of biosorption process using goat teeth for trapping heavy metal ions.
Materials and Methods
Goat teeth were collected from a local abattoir and were detached from the strongly bounded jaw bone. Macroscopic impurities like soft tissues/ bone marrow associated with teeth were removed carefully under running water and dried in the sunlight for a period of ten days. The preliminary screening batch studies were performed with raw, pre-cleaned goat teeth (RGT). It is pulverized in a electrical mixer and sieved using Scientifically Tested Molecular Sieves (JAYANT Scientific Instruments Co., Mumbai) to assess the efficiency of the untreated material (GT). The pictorial representation of (RGT) and pulverized material (GT) are shown in Fig 2.
Preparation of Biosorbent
Goat teeth were rinsed with distilled water and air dried followed by soaking in acid 0.1 N HCl for 4 h at room temperature. The Treated Goat Teeth (TGT) was subjected to several washings with doubly distill water until a pH 7.0 ± 0.5 was attained (Labtronics Deluxe pH meter), air dried, powdered using electrical mixer and categorized to the desired particle sizes.
Characterization Studies
The physicochemical characteristics of the modified adsorbent were performed for 0.18 mm particle size. The parameter experimented are, pH (ELICO (LI-120) pH meter); Moisture content (Xylene-extraction test method (ASTM D 2867-95)); Bulk Density (Specific gravity bottle); Specific Gravity (Pycnometer with Thermometer); Ash Content (Yorco -Muffle Furnace).ing heavy metal ions.
Surface Morphological Studies
The treated and Pb(II) loaded TGT were subjected to spectral analyses (within the range of 400-4000 cm-1) to determine the main functional groups present in the sorbents using Shimadzu Infrared Spectrophotometer. The surface morphology of the treated goat teeth before and after Pb(II) sorption was examined using JEOL JFM- 6390 Scanning Electron Microscope (20 kV) under vacuum of 1.33 x10-6 m Bar. The samples were covered with a thin layer of platinum (10 nm) using a sputter coater (SCD 0050 – Baltec, Liechenstein) and subjected to scanning for the determination of the chemical composition. Energy dispersive X-Ray analysis was performed for the adsorbents to estimate the element composition.
Metal Solution - Preparation
All the reagents employed for the experimental setup were of Analytical Grade. The solutions were prepared using doubly distilled water. A stock solution of 1000 mg/L was prepared by dissolving 1.598g of Pb(NO3)2 in a 1 litre standard flask. Standard solutions of varied concentrations were prepared from the stock and further aliquots were done by respective dilutions as and when required. pH environments were adjusted by and large using 0.1N HCl / 0.1 N NaOH.
Batch Studies
Equilibration studies were carried out in 250 ml iodine flasks, with 50 ml of sorbate species at variable dosage of TGT (0.1, 0.2, 0.5 and 1 g), in a rotary shaker(KEMI) to describe the role of variables viz., particle sizes (0.18mm, 0.21mm, 0.30mm, 0.42mm and 0.71mm), contact time (5, 10, 30, 60 min), initial metal ion concentration (100-1000: 250mg/L interval) and pH (1,3,5,7 and 9).
Desorption / Regeneration Studies
Following batch studies, a pilot column was run to check the desorption / regeneration capacity of the chosen material at a flow of 200ml / 5min with a load of 40mg using 0.1N HCl as the desorbing agent.
Data Analysis
The initial and residual Pb(II) ion concentrations were recorded using AAS (Shimadzu 6200 AA Atomic Absorption Spectrophotometer) at a wavelength of 283 nm. The percentage values and the amounts of Pb(II) adsorbed from aqueous solutions were calculated using the equations, % adsorption = (Ci – Ce) / Ci ËŸ 100 and q = V(Ci - Ce) / W respectively.
Experimental Verification Studies
The mathematical isotherm models developed by Langmuir, Freundlich, Tempkin and Dubinin- Kaganer- Radushkevich were applied to the equilibrium data in order to understand the sorption mechanism. The efficiency of the adsorbent was evaluated by following adsorption kinetics viz., pseudo- first order, pseudo-second-order. The calculation was made for the changes in standard free energies, enthalpies and entropies of the adsorption systems to determine the possibility and feasibility of the sorption reactions. The extent of statistical fit was verified using the output variable tools viz., ANOVA, Pearson Correlation and descriptive analysis with significance based on 95% confident level using IBM SPSS Statistics 20 software.
Results And Discussion
Screening Study
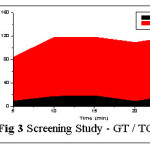
Sorptive ability of Goat Teeth (GT) and HCl treated GT (TGT) were screened separately. An increase in the biosorption of Pb(II) ions was observed using TGT against RGT (fig 3). This could be due to more exposure of active metal binding sites, after subjecting the material to acid treatment.7
Physiochemical Characteristic Study
Table 1 reveals the physiochemical characteristic study of TGT affecting the adsorptive capacity. TGT exhibits neutral pH value. Low moisture content value implies stable nature with seldom storage problem of the prepared material.8 Ash content value (≈ 1%) favor the presence of scrimpy inorganic matter with abundant carbon content leading to the availability of greater adsorption sites. Specific gravity value < 2.0 indicate that adsorption onto organic material is inactive during complex formation.9 Bulk density value <1.2 favors the presence of fine particles with higher pore volume. The porosity represents the micro pore content of the material. High Ca/P ratio proves that TGT is a non- stoichiometric Hydroxyapatite (ie., > 1.67).10
Table 1: Physic-chemical Characteristics
|
Properties |
TGT |
|
pH |
7.28 |
|
Moisture Content (%) |
1.89 |
|
Ash Content (%) |
1.03 |
|
Specific Gravity |
1.62 |
|
Bulk Density (g/cm3) |
0.59 |
|
Porosity |
63.7 |
|
Element Composition(%): Ca |
29.3 |
|
P |
14.6 |
|
Ca/P |
1.99 |
|
O |
56.0 |
Table 2: Physic-chemical Characteristics -Comparison
|
Bone Wastes* |
Moisture Content (%) |
Ash Content (%) |
Bulk Density (g/cm3) |
|
TGT |
1.89 |
1.03 |
0.59 |
|
CBAC 11 |
3.9 |
10.75 |
2.56 |
|
DBAC11 |
4.5 |
19.00 |
2.24 |
|
GBAC11 |
5.4 |
26.75 |
2.32 |
|
CHBAC11 |
97 |
23.75 |
2.32 |
A comparison of the physiochemical characteristics of various activated carbons prepared from bone wastes11 [Cow Bone Activated Carbon(CBAC), Dog Bone Activated Carbon (DBAC), Goat Bone Activated Carbon (GBAC) and Chicken Bone Activated Carbon (CHAC)]* made with TGT (Table 2) reveal that the latter possess lower moisture and ash content leading to enhanced active metal binding sites. Lower bulk density values support the large surface area with many pores.
 |
|
 |
|
 |
|
 |
|
IR Study
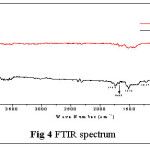
The FT-IR spectra of both treated and Pb(II) loaded TGT systems are represented in Fig. 4. The overlapping peaks between the regions 3000- 3700 cm-1 correspond to the stretching vibrations of structural OH- group in Calcium phosphate hydroxy apatite. The characteristic peaks at 1013 cm-1 and 556 cm-1 can be assigned to Ê‹3 asymmetric P-O stretching and Ê‹4 O-P-O bending modes respectively of PO43-group.12 The low intensity peaks at 1744 cm-1, 1668 cm-1/ 1515 cm-1, 1217 cm-1 refer to the bending vibrations of OH- / CO32-groups. The libration mode of O-H vibration is seen at 613 cm-1. The peak at 867 cm-1 could be referred to HPO42- group.13 Pb(II) loaded TGT spectrum has less number of peaks and rise in the peak intensity compared to the unloaded TGT due to metal sorption.
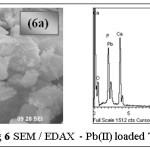
SEM / EDAX Analysis
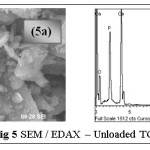
The surface morphologies of the treated and loaded samples were analyzed using SEM and EDAX. Irregular coarse surface texture of fig.5a correspond to the unloaded TGT, where surface morphology has undergone specific changes with large flake-like cluster arrangement in the case of metal loaded SEM image (fig 6a). Fig. 5b, 6b record the EDAX spectra of TGT and Pb(II)-TGT respectively, where the presence of new peak at 12.8 keV correspond to the effective adsorption of Pb(II) onto TGT, apart from Ca, P, O being major constituents as indicated.
 |
|
 |
|
 |
|
 |
|
 |
|
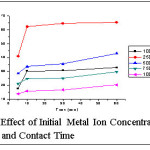
Effect of Initial Metal Ion Concentration and Contact Time
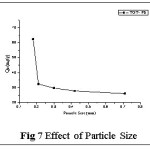
The sorbent particle size plays a crucial role in biosorption phenomenon of sequestering metal ion from aqueous solutions. The experimental results for the adsorption of Pb(II) at five different particle sizes of TGT (0.18mm, 0.24mm, 0.30mm, 0.41mm and 0.71mm) depicted in fig 7, revealed that a maximum sorption of (62.32mg/g) for the smaller particle size of 0.18mm. This can be attributed to the fact that smaller particle size offers a larger surface area with functional groups and having tendency to equilibrate in a shorter time.14 Thence, 0.18mm particle size is fixed as optimum size for the forthcoming experiments.
Effect of Particle Size
Initial metal ion concentrations in the range of 100-1000 mg/L: 250 mg/L at varied agitation time intervals (5, 10, 30, 60) is represented in fig 8. The amount of metal uptake per unit weight of TGT is (53.59 mg/g to 62.32 mg/g) linearly increased from 100 to 250 mg/L ie., implying the set of concentration gradient. The uptake of heavy metal ions by sorbents has often been observed to occur in two stages. The two stage sorption are first rapid/quantitatively predominant and the second quantitatively insignificant.15
The initial uptake rate was very rapid later gradual indicative of an equilibrium state attainment at 10 min, aftermath reading a plateau. This result reflects the fixing of initial metal concentration and contact time as 250 mg/L and 10 min respectively.
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
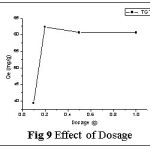
Effect of Dosage
The cumulative rise in the qe values with increasing dosages (0.1- 1.0 g) is evidenced from Fig 9 wherein the sorption rate is significantly higher (62.32 mg/g) for 0.2g/50ml. The continuous uptake of metal ion on the biosorbent matrix might be due to the availability of larger metal binding sites for the complexation of the heavy metals. 0.2g dosage was optimized for further studies.
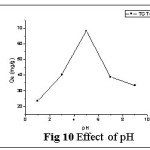
Effect of Solution pH
pH of the aqueous solution is an important parameter and it may change or control the solubility/ degree of rapid ionization of metal ions and concentration of counter ions on the adsorbent.16 The the role of H+ ion concentration was determined on the Pb(II) removal at varied pH environments (1- 11). Fig 10 shows a random increase in metal sorption from pH 1-5, owing to the more competitive adsorption between H+ and Pb(II) ions. Removal efficiency attained its own maximum at pH 5 due to lesser H+ ion concentration. pH 6 is the point of formation and precipitation of metal hydrolysis and this occurs by the replacement of central metal ligands in the inner coordination sphere with hydroxyl group.
At pH > 6, the presence of oxygen containing functional groups, conferred negative charge on the adsorbent (TGT) surface and provide less active sites for Pb(II) sorption due to the occurrence of repulsive electrostatic interactions. Comparative studies on the pH/ Adsorption capacity with other researchers’ material are presented in table 2. TGT, a novel material identified is found to possess excellent capacity as a treated one amongst the activated carbons of other researchers.
Table 3: Comparison of the adsorption capacity (Qe) of various heavy metals on several adsorbents
|
pH |
Adsorbent |
Metal Ion |
Adsorption Capacity (mg/g) |
Reference |
|
5.1 |
Cow Bone Charcoal |
Ni (II) |
32.5 |
[17] |
|
Cu(II) |
35.44 |
|||
|
Fe(II) |
31.43 |
|||
|
Pb(II) |
19.92 |
|||
|
5.5 |
Bone Powder |
417 |
[18] |
|
|
Bone Char |
1828 |
|||
|
Activated Bone Char |
690 |
|||
|
Fish Bone |
323 |
[19] |
||
|
Commercial Bone Char |
18 |
|||
|
3 |
Bovine Bone Ash |
Cr(VI) |
1.4684 |
[20] |
|
Billy Goat Bone |
Zn(II) |
23.64 |
[21] |
|
|
Bovine Bone |
20.747 |
|||
|
5.6 |
Chicken Bone ash |
Cd(II) |
1924 |
[22] |
|
Ni(II) |
1854 |
|||
|
Zn(II) |
1627 |
|||
|
Pb(II) |
1842 |
|||
|
5 |
HCl modified Goat teeth |
62.32 |
Present Study |
Isothermal Studies
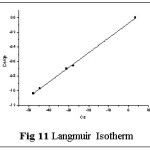
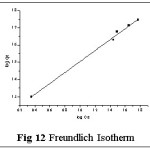
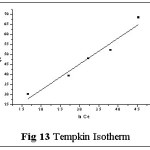
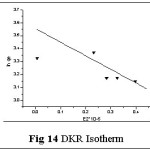
Isotherms give an expression for encompassing the surface nature and the exponential distribution of active sites with their energies. Four empirical models viz., Langmuir, Freundlich, Tempkin and DKR isotherms were applied to the sorption system of Pb(II) - TGT at varied initial metal ion concentrations (100- 1000 mg/L). These isotherms relate metal uptake per unit weight of the adsorbent qe against the equilibrium metal ion concentration in the bulk phase Ce. The isothermal equations, sequential plots and corresponding constants involved are listed in Table 4. The linear plots are arrived based on the concentration values correspondingly depicted in figs: 11-14.
Table 4: Adsorption Isotherms
|
Isotherm Model23 |
Linear Equation |
Plot |
Parameters |
|
Langmuir |
Ce/qe = Ce/qm +1/bqm |
Ce vs Ce/qe |
b, qm |
|
Freundlich |
log qe = log KF + 1/n log Ce |
log Ce vs log qe |
KF, 1/n |
|
Tempkin |
qe = BT ln AT + BT ln Ce |
ln Ce vs qe |
AT , BT |
|
DKR |
ln qe = ln qs -bDR e |
e2 vs ln qe |
qs,E |
The corresponding Isothermal constant values / correlation coefficients derived from the graphical representations were calculated and are listed in table 5. The constants qm, KF and Qs correspond to sorption capacities calculated from the slope values of Langmuir, Freundlich and Dubinin- Kaganer- Radushkevich (DKR) plots respectively. b, BT represent the heats of adsorption and 1/n show the sorption intensity correspondingly. AT and E relates to the respective equilibrium binding constant and mean free energy.
Table 5: Isothermal Parameters
|
Langmuir |
Freundlich |
Tempkin |
DKR |
||||
|
qm (mg/g) |
49.5049 |
KF (mg/g) |
15.3532 |
AT (L/g) |
1.9056 |
qs (mg/g) |
33.3064 |
|
b (L/mg) |
0.2704 |
1/n |
0.3176 |
BT (J/mol) |
12.839 |
E (KJ/mol) |
9.4675 |
|
R2 |
0.999 |
R2 |
0.9959 |
R2 |
0.9863 |
R2 |
0.7747 |
Correlation coefficient (R2) pertaining to the studied isotherms are observed as: Langmuir > Freundlich > Tempkin > DKR indicating Langmuir model to best describe the monolayer adsorption appear to be consistent with the specific sites. qm value being greater than Kf and qs values indicate better sorption capacity, supporting Langmuir model, even though linear fit is obvious in case of Freundlich isotherm. Also, sorption intensity value lying within 0.1< 1/n <1.0 indicates favorable adsorption. Monolayer adsorption is favoured by the AT and BT values (Tempkin Constants). Mean free Energy value derived from DKR isotherm plot being 8 < E < 16 KJ/mol-1, indicate ion exchange explains the sorption mechanism.23,24
Kinetics Studies
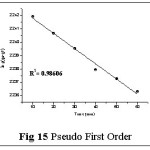
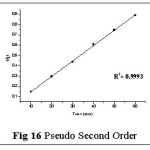
Kinetic studies play a greater role in the removal of metal ions from the aqueous solution. The kinetic equations, deduction of relative values to draw consecutive plots and their constants are shown in Table 6.
Table 6: Adsorption Kinetics
|
Model |
Equation |
Plot |
Constants |
|
Pseudo First Order |
log(Qe- Qt) = log Qe - Kf t / 2.303 |
log(Qe- Qt) vs t |
qe, Kf, R2 |
|
Pseudo Second Order |
t/Qt = 1/(Ks Qe2) + (1/Qe) t |
t/Qt vs t |
qe, Ks, R2 |
The sorption rate constants of Pb(II)– TGT system (Table 7) were calculated from time profiles with respect to the concentration gradients and the kinetic (Pseudo-first order and Pseudo-second-order) plots are depicted in figs: 15 and 16. The calculated linear correlation coefficient value (R2) is relatively higher for pseudo second order model as obvious from the best linear fit (Fig 16), the corresponding sorption capacity (67.1140 mg/g) matching with the experimental value (62.32 mg/g) derived from the data analysis of Batch Study (Page No 5). This facilitates the better description of the adsorption system by pseudo second order kinetic model.25
Thermodynamic Studies
The adsorption mechanism can be determined via thermodynamic factors viz., change in free energy (∆G0) was calculated using equations: ΔG = -RT ln Kc. Thermodynamic constants are calculated using the linear regression analysis and listed in table 8. The thermodynamic parameters enthalpy of adsorption (∆H0) and entropy (∆S0) were calculated from the slope and intercept of Vant Hoff’s plot respectively.The negative enthalpy (∆H0) value implies the energetically favorable adsorption process.26 The negative ∆G0 values shows all the interactions between metal ions with adsorbent are energetically feasible and spontaneous process. The magnitude of DG0 was directly proportional to temperature, implying that the sorption is more favorable at higher temperatures. The positive value of ∆S0 from table 8 suggests the increased randomness at the solid-solution interface during adsorption.
Table 7: Kinetic Parameters
|
Kinetic Model |
Parameter |
Value |
|
Pseudo-First-Order |
Kf x 10-2 (min-1) |
0.23 |
|
qe, cal (mg g-1) |
174.9 |
|
|
R2 |
0.986 |
|
|
Pseudo-Second-Order |
Ks x 10-2 (g mg-1 min-1) |
7.20 |
|
qe, cal (mg g-1) |
67.11 |
|
|
R2 |
0.999 |
Table 8: Thermodynamic Parameters
|
Temp (K) |
Parameter |
Value |
|
273 |
∆G0 (kJ mol-1) |
-1.1858 |
|
293 |
-1.8964 |
|
|
303 |
-2.2573 |
|
|
313 |
-2.4783 |
|
|
323 |
-3.0333 |
|
|
333 |
-3.4773 |
|
|
∆H0 (kJ mol-1) |
-10.976 |
|
|
∆S0 (J mol-1 ) |
45.293 |
Desorption / Regeneration Studies
The result of the pilot column reveals that the Pb(II) ions have been immobilized on the surface of the sorbent material, thereby no desorption of the divalent ion was observed, after running the column with 0.1N HCl. Moreover, TGT was found to possess 100% regeneration capacity for 5 cycles of repeated sorption. On completion of exhaustion, the saturated TGT can serve as a fertilizer for plants27
Statistical Analysis
A statistical basis calculation (Table 9) supports the experimental values for error free adsorptive environment. Descriptive, Correlation and ANOVA studies were performed to assess significant differences among the four factors viz., particle size, dosage, metal concentrations and pH. A value of P<0.01 was considered as significant. The calculated positive Kurtosis and Skewness values correspond to the peak and degree of asymmetry in the distribution. The Pearson correlation analysis shows the relationship between the dataset. The factors except particle size are positively correlated with experimental data. The assessment of variance (P and F) values were verified using One way ANOVA at 95% confidence level, the P values being <0.01 and F > Fcrit-value favor the data to be statistically significant i.e., the rejection of null hypothesis is observed in all parameters except dosage.
Table 9: Descriptive / ANOVA/ Correlation studies
|
Particle Size |
Dosage |
Initial Concentration |
pH |
|
|
Mean |
36.8551 |
57.2022 |
64.5248 |
40.8182 |
|
Standard Error |
7.9200 |
6.21633 |
2.2217 |
7.4770 |
|
Median |
29.7481 |
60.6027 |
67.2559 |
38.926 |
|
Standard Deviation |
17.7097 |
12.4326 |
7.6962 |
16.7192 |
|
Kurtosis |
4.64047 |
2.7723 |
9.4137 |
2.6029 |
|
Skewness |
2.1371 |
-1.4773 |
-2.9713 |
1.3256 |
|
Pearson Correlation (r) |
-0.5844* |
0.3662* |
0.3630* |
0.1765* |
|
F |
20.9286 |
3.8618 |
43.4138 |
23.6821 |
|
P |
0.001739 |
0.1441 |
3.9223E-05 |
0.0082 |
|
F crit |
5.3176 |
10.1279 |
4.8443 |
7.7086 |
*Correlation is significant at the 0.01 level (2-tailed)
Conclusion
Goat teeth, a solid waste dumped in local butcher’s shop were collected, treated and subjected to equilibration studies to evaluate its trapping ability of Pb(II) ions. The batch studies indicated 100% removal on Pb(II) by TGT at optimized condition of 0.18 mm sorbent’s particle size, 250 mg/L initial metal concentration, 0.2 g sorbent dosage, 10 min equilibration time and pH 5 at room temperature (293K). Physic-chemical parameters, FT-IR, SEM/ EDAX analyses of treated and metal laden TGT registered the whole mass adsorption process. The sorption data fitted were into Langmuir, Freundlich, Temkin and Dubinin- Kaganer- Radushkevich isotherms amongst which Langmuir Adsorption model was found to possess the highest regression value compared to other isotherms. The sorption kinetic studies followed Pseudo second order mechanism. Thermodynamic calculations indicated the feasibility, exothermicity and spontaneity of the adsorption process. Though not carbonized, the chemically modified goat teeth possessed excellent sorption capacity (62.32 mg/g) in the removal of Pb(II) ions. No leaching out of the metal ions from the exhausted TGT was observed with 0.1N HCl as the desorbing agent. Regeneration capacity of the metal loaded material was recorded as 100% for 5 cycles of repeated sorption. Thus, it is concluded that this inexpensive material is promising adsorbent for trapping heavy metals from laboratory aqueous solutions. The future scope of this work lies in the initiation of scaling up the adsorption process to industrial wastewaters.
Reference
- Bibudhendra Sarkar. Heavy Metals in the Environment. CRC Press, 2002
CrossRef - Rajeev Bhat, Vicente M. Gomez-Lopez, Practical Food Safety: Contemporary Issues and Future Directions John Wiley & Sons (2014)
- Lanphear BP., Dietrich K., Auinger P., Cox C., Cognitive deficits associated with blood lead concentrations < 10 microg/dL in US children and adolescents. Public Health Rep. 115(6):521-9 (2000)
CrossRef - Lawrence K. Wang, Norman C. Pereira. Solid Waste Processing and Resource Recovery.
- Springer Science & Business Media (2012) NTP Monograph on Health Effects of Low-Level Lead, National Institute of Environmental Health Sciences, June 13 (2012)
- Ai Phing Lim, Zufarzaana Zulkeflee and Ahmad Zaharin Aris. Effective removal of lead (II) ions by dead calcareous skeletons: sorption performance and influencing factors. Water Science & Technology, 74(7) 1577-1584 (2016)
CrossRef - Muhammad Aqeel Ashraf., Mohd. Jamil Maah., and Ismail Yusoff. Removal of Lead from Synthetic Solutions by Protonated Teleosts Biomass. E-Journal of Chemistry, 9(1), 345-353 (2012)
CrossRef - Malik, D.S., Jain, C.K., and Anuj K.Yadav. Preparation and Characterization of Plant Based Low Cost Adsorbents, Journal of Global Bioscience, 4(1), 1824-1829 (2015)
- Michael A. Callahan, Water-related environmental fate of 129 priority pollutants, Office of Water Planning and Standards, Office of Water and Waste Management, U.S. Environmental Protection Agency (1979)
- Agnieszka Sobczak, Zygmunt Kowalski, and Zbigniew Wzorek. Preparation of Hydroxyapatite from Animal Bones. Acta of Bioengineering and Biomechanics, 11(4):23-29 (2009)
- Abubakar Mohammed, Alechenu A. Aboje, Manase Auta and Mohammed Jibril. A Comparative Analysis and Characterization of Animal Bones as Adsorbent. Advances in Applied Science Research, 3(5):3089-3096(2012)
- Bayram Kizilkaya, and Adem TekJnay A. Utilization to Remove Pb (II) Ions from Aqueous Environments using Waste Fish Bones by Ion Exchange. Journal of Chemistry, 1- 12 (2014)
- Meski S., Ziani S., and Khireddine H. Removal of Lead Ions by Hydroxyapatite Prepared from the Egg Shell. Journal of Chemical Engineering Data, 55:3923–3928(2010)
CrossRef - Ajay Oraon, Neha Keshri and Amit Kumar Gupta. Comparative Study of Biosorption Capacity of Cadmium on Banana Peel and Cactus for Different pH and Particle Size. International Journal of Innovative Research in Science, Engineering and Technology, 5 (11):19302- 19305(2016)
- Ejikeme, Ebere M., Ejikeme, P. C. N., Abalu, Benjamin. N. Equilibrium, Kinetics and Thermodynamic Studies on MB Adsorption Using Hamburger Seed Shell Activated Carbon. International Journal of Engineering & Technology 14(03), 74- 83 (2014)
- Amuda O.S., Giwa A.A., and Bello I.A. Removal of Heavy Metal from Industrial Wastewater using Modeified Activated Coconut Shell Carbon. Biotechnology Engineering Journal, 36,174- 181(2007)
- Juan Carlos Moreno, Rigoberto Gómez and Liliana Giraldo. Removal of Mn, Fe, Ni and Cu Ions from Wastewater Using Cow Bone Charcoal. Materials, 3, 452-466 (2010)
CrossRef - Sudaratn Lurtwitayapont and Thares Srisatit. Comparison of Lead Removal by Various Types of Swine Bone Adsorbents. Environment Asia, 3(1), 32-38 (2010)
- Didilia I. Mendoza-Castillo, Adrián Bonilla-Petriciolet, Juan Jauregui-Rincon and Merced Martínez-Rosales.J. Removal of Lead Metal Ions From Water by Bone Char. Indian Journal of Science and Technology, 8(13) (2015)
- Faezeh Tadjik, Omid Mirzaee and Homeira Ebrahimzadeh. Characterization of Cr(VI) Removal from Aqueous Solutions by Natural Hydroxyapatite from Bone Ash and Determination of Optimum Conditions. Indian Journal of Science and Technology, 8(13), 1-7 (2015)
CrossRef - Meskia S., Khireddinea H., Ziania S., Rengarajb S and Mika Sillanpaab. Comparative Study on the Removal of Zinc(II) by Bovine Bone, Billy Goat Bone and Synthetic Hydroxyapatite. Desalination and Water Treatment, 16, 271–281(2010)
CrossRef - Alisson Gomes Paulinoa, Adriana Jesus da Cunhaa, Rení Ventura da Silva Alfayaa & Antonio Alberto da Silva Alfayaa. Chemically Modified Natural Cotton Fiber: a Low-Cost Biosorbent for the Removal of the Cu(II), Zn(II), Cd(II)and Pb(II) from Natural Water. Desalination and Water Treatment, 52, 4223–4233(2014)
CrossRef - Dada A.O., Olalekan A.P., Olatunya A.M., Dada O, Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ onto Phosphoric Acid Modified Rice Husk. IOSR Journal of Applied Chemistry, 3(1), 38-45(2012)
CrossRef - Manal El-Sadaawy, Ola Abdelwahab. Adsorptive Removal of Nickel from Aqueous Solutions by Activated Carbons from Doum Seed (Hyphaenethebaica) Coat. Alexandria Engineering Journal, 53, 399–408(2014)
CrossRef - Ramesh S. T., Rameshbabu N., Gandhimathi R., Nidheesh P. V., and Srikanth Kumar M. Kinetics and Equilibrium Studies for the Removal of Heavy Metals in Both Single and Binary Systems using Hydroxyapatite. Applied Water Science 2:187–197(2012)
CrossRef - Friday O. Nwosu, Bamidele I. Olu-Owolabi and Kayode O. Adebowale. Kinetics and Thermodynamic Adsorption of Pb(II) and Cd(II) Ions from used Oil onto Thevetia neriifolia Nutshell Active Carbon. Current Research in Chemistry, 4(2): 26-40 (2012)
CrossRef - Anne Boen and Trond Knapp Haraldsen. Meat and bone meal and biosolids as slow release phosphorous fertilizers. Agricultural and Food Science, 22(2): 235- 246 (2013)







