Assessment of lowest chromium bioaccumulation vegetables irrigated by Sheba Leather Industry contaminated water in Wukro, Tigray – Ethiopia
Corresponding author Email: abrishkw2011@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.15.1.14
High chromium concentration is threatening to the environment, since it is persistent and non-biodegradable pollutant while introduced once to our planet. Bioaccumulation capacity of chromium has carcinogenic potential to human beings. To minimize the risk of chromium bioaccumulation, the study was conducted in Wukro irrigation fields. 54 plants, 24 water and 18 soil samples were collected in triplication from the selected points of upstream, treatment plant and downstream of the Sheba leather industry. Samples were transported to advanced laboratory for chromium analysis and results were analysed by R-software. Downstream vegetable samples were measured higher chromium bioaccumulation compared to the upstream sites. Highest chromium concentration was recorded in rood edible vegetables, and the lowest was measured in fruit edible vegetables. Chromium concentration was significantly different between the upstream and downstream vegetables, water and soil samples at p<0.01, p<0.05 and p < 0.001 respectively. The chromium bioaccumulation order were root edible > leaf edible > fruit edible vegetables. Environmental laws have to be strictly enforced and further researches are recommended.
Copy the following to cite this article:
Weldemariam A. K. Assessment of lowest chromium bioaccumulation vegetables irrigated by Sheba Leather Industry contaminated water in Wukro, Tigray – Ethiopia. Curr World Environ 2020; 15(1).
DOI:http://dx.doi.org/10.12944/CWE.15.1.14Copy the following to cite this URL:
Weldemariam A. K. Assessment of lowest chromium bioaccumulation vegetables irrigated by Sheba Leather Industry contaminated water in Wukro, Tigray – Ethiopia. Curr World Environ 2020; 15(1). Available from: https://bit.ly/3aKPO7C
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 20-02-2020 |
|---|---|
| Accepted: | 30-03-2020 |
| Reviewed by: | 
 Chadetrik Rout
Chadetrik Rout
|
| Second Review by: |

 Khadar Babu
Khadar Babu
|
| Final Approval by: | Dr Umesh Kulshrestha |
Introduction
Untreated and semi-treated effluents from tanneries and other industries are often discharging in to the environment, particularly in developing countries. Farmers’ are ignorance about the hidden toxicity of such heavily polluted discharges and their subsequent negative impacts has led to its continued use in cultivation of cereals, vegetables and others economically important plants.1 The discharge from various sub-processes in tanneries like bathing, pickling, tanning, dyeing and fat liquoring may cause severe water pollutions, mainly that of chromium. The pollution of water bodies is very often linked to an industry or sewage or agricultural runoff.2 Such, contamination of soil by the release of heavy metals from industrial or other anthropogenic activities are a threat to human health and ecosystem. Presence of heavy metals in soil is known to have potential toxic impact on environmental quality and on human health via water, vegetables and various crops.3, 4 In addition, it may cause cancer is reported bioaccumulation of heavy metals in human beings through food chains.2, 7 Concentration of heavy metals in soil may adversely affect soils productive because of phyto-toxicity, and previous works have shown inhibition of seed and shoot germination, especially because of chromium (Cr6+).5, 6
Tannery effluents are ranked as containing the highest pollutants among all the industrial wastes. In Ethiopia alone it estimated that about 200-300 tons of chromium is released into the environment annually from tannery industries. Chromium concentrations ranging between 2000 and 5000mg/l in the aqueous effluents compared to the recommended permissible limits of 2mg/l are contributing from tanning process in leather industries. Chrome tanning is the main contributor of chromium pollution in Ethiopia. Chrome Tanning process is most common in Ethiopia, which is discharging high chromium levels to the environment.8 Leather industry requires large amount of different chemicals, but one of the major emerging environmental problems in the tanning industry is the disposal of chromium contaminated wastes to the environment.9, 10 Chromium exists in oxidation states of +2, +3, and+6. The trivalent oxidation state is the most stable form of chromium and is essential to mammals in trace concentration and relatively immobile in the aquatic system due to its low water solubility.11 The hexavalent chromium is much more toxic to many plants, animals, and bacteria inhabiting aquatic environments.11
The study was conducted at Wukro irrigation field, which is high potential and crucial area to cultivate various vegetables; Onion (Allium cepa), Potato (Solanum tuberosum), Carrot (Daucus carota), Lettuce (Lactuca sativa), Cabbage (Brassica oleracea), Spinach (Spinacia oleracea), Green Pepper (Capsicum annuum), Tomato (Solanum lycopersicum) and Maize (Zea mays), etc. However in this irrigation fields there is Sheba leather industry P.L.C with weak waste managements; as the result there is alarming chromium concentrations in the irrigation fields.11 This study was designed to assess lowest chromium bioaccumulation vegetables in their edible parts only.12 These selected vegetables are common and most dominant in our daily dishes.
Material and Method
Description of Study Area
The Wukro town is located in TRNS and is about 45km and 825km from Mekelle and Addis Ababa, respectively and located in eastern Tigray with 13o78'33''N latitude, 39o60'00''E longitude.13 Sheba leather industry having a capacity of processing 6000 hides a day is located in Wukro irrigated areas.14
Experimental Design
The vegetable cultivation area is characterized by summer rainfall pattern, with peak rainfall during June to September. So to avoid the influence of rain water the study was conducted in the dry season to evaluate the effect of Sheba leather industry wastes on vegetables cultivating in the area. Experiment was conducted on 1meter x 1meter area by transplanting vegetables upstream and downstream of the sites of Sheba leather industry, duration of January to March 2018. The upstream site was considered as it is control was expected to be free from the industry waste, while the downstream site was assumed to be contaminated industry’s wastes. Different vegetable types were cultivated made mixed cropping and transplanting at the upstream and downstream sites with similar species, patterns and at the same time.12, 15 The vegetables were raised in a nursery and transplanted into the prepared sites at the same time. The upstream vegetables were adequately irrigated with spring water, whereas the downstream were irrigated with the industry waste contaminated river water. There was no fertilizer and pesticides application for protection against insect.15
Sample collection and preparation
Water Sampling
The spring and waste contaminated irrigation water samples were collected in 250ml polyethylene bottles. Eight sampling point were selected, out of which two from WWTP (influent and effluent). Each of the eight sampling point was sampled in triplicate resulted in 24 samples; each triplication was mixed and homogenized in to the representative sample before analysis. The physical water quality parameters were measured on site, for Temperature, pH, EC, DO, and TDS during sample collection. Water and wastewater samples were acidified on site, with 3ml of concentrated HNO3 to avoid microbial growth and to minimize chromium precipitations; samples were kept in Icebox and transported for chromium analysis.11
Vegetable sampling
Sampling was done in triplicate for the edible parts of each of the nine vegetables for the upstream and downstream sites separately. The samples were deducted by hand, washed with clean tap and distilled water to remove the soil particles and placed in clean plastic bags. The samples were brought to Ezana laboratory and kept in a refrigerator before oven drying and digestion. The samples were analysed for chromium by AAS.11
Soil Sampling
Mixed top soils (0-15cm), medium depth soils (15-30cm) and depth soils (30-45cm) samples were collected into clean plastic bags using a stainless-steel auger from the selected upstream and downstream fields, where the vegetables were cultivated. Each of the two sites and each of these depths were sampled in triplicate, air-dried at room temperature (approximately 25°C) and powdered (by a mortar and pestle); followed by oven drying (105oC for 12hr). The samples were analysed for total chromium using AAS instrument.11, 14
Digestion of samples for chromium analysis
Water samples digestion and analysis
The collected each triplicate water and wastewater samples were mixed and homogenized thoroughly into one representative samples and 50ml of each such sample was transferred to separate conical flask. 10ml of 68%HNO3 was added to each flask and was covered and digested on a hotplate for 6hrs at 100oC. The solution was then transferred to 100ml volumetric flask and diluted up to the mark with distilled water and mixed thoroughly. 20ml of final solution was used to measure chromium on AAS.14
Vegetable samples digestion and analysis
Homogenized, dried and powdered vegetable samples 1g were taken and transferred to 100ml beaker. 5ml of 68%HNO3 and 2ml of 70%HClO4 were added and the solutions were heated for 45minutes (until yellow colour changed to white smog) at 100 oC. Filtered into 50ml volumetric flask and diluted up to the 50ml mark with distilled water and mixed, allowed to settle and was measured chromium using AAS.11, 14
Soil samples digestion and analysis
A sample of 20+0.05g of powdered (75μm) soil was weighed in a 400ml tall form beaker. An acid mixture of 50ml of 37%HCl and 20ml of 68%HNO3 was slowly added to the sample while swirling, on the hot plate for a minimum of 45minute at 160 OC, stirring with a glass rod. It was removed from the hot plate before becoming dry, cooled and diluted up to 200ml mark of volumetric flask with distilled water, shaken and poured back in to the beaker and allowed to settle for 30minnute. Finally, samples were measured for chromium on the AAS.14
Quality assurance and data analysis
Standard chromium reference reagent was used for calibration and quality assurance for each analytical batches, and chromium standard reagent was spiked to check precision and accuracy.12 To analysis the result R-software (Rx64 3.4.4 version) was used. All data were normally distributed (p>0.05), except that of EC, TDS and Cr results in the WWTP.12
Result and Discussion
Environmental effects of Sheba leather industry wastes
Physico-chemical of Irrigation water quality parameters
Temperature, pH, EC, DO and TDS, were measured on site during sample collection and the result was temperature 23.27±1.460c, pH 7.86±0.71, EC 6950±4368 µS/cm and DO 5.61±1.78 mgL-1. In addition, average Cr was 6.96±7.04 mgL-1(Figure 3‑1). EC, pH and temperature and chromium were shown significant difference between the controlled and contaminated sites (p<0.05), while not significant for DO and pH. Highest EC, TDS and Cr were recorded in the sampling point nearest to Sheba leather industry and lowest were recorded away from the industry wastes. This suggested that, Sheba leather industry may pollute to the environmental.14, 16
Chromium concentration in soils
Chromium concentration was higher in the downstream, with average value of 32.18±0.70; whereas the lower was measured in the upstream, with the value of 20.45±1.89 (Figure 3‑1). The chromium concentration was shown significant difference between downstream and upstream soil sites (p<0.001), in the other hand not significantly different between their soil depths (Figure 3‑1). This indicated that, there may high contribution of chromium pollutant from the leather industry to the soil. In addition, chromium measured in the upstream soils were high compared to natural soils; this could be the selected cultivation site was down of Wukro town.11, 14
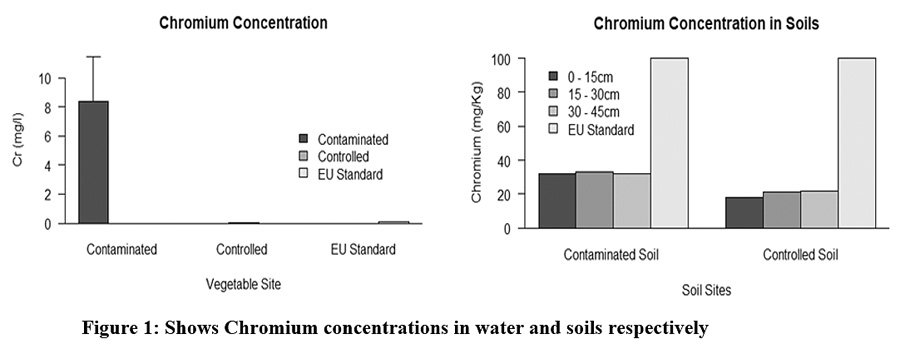 |
Figure 1: Shows Chromium concentrations in water and soils respectively Click here to view Figure |
Chromium concentrations in vegetables
Higher chromium concentration was measured in vegetables which were cultivated in the downstream while lower chromium bioaccumulation was recorded in the upstream (Figure 3‑2). The highest chromium bioaccumulation was recorded in root edible vegetables, whereas the lowest was in the fruit edible vegetables (Figure 3‑2). The average value of chromium concentration in vegetables cultivated in upstream was 1.76±0.68 and downstream 3.19±1.60. The chromium bioaccumulation of vegetables was significant difference between upper and downstream sites (p<0.05), and chromium bioaccumulation was significantly different (p<0.005) in their edible types (Figure 3‑2). In the other side, no significant difference among these all vegetable species. Thus, high chromium bioaccumulation concentration was recorded in the downstream and might be contribution of the leather industry wastes to the environment.11, 14
Evaluation efficiency of Sheba leather industry WWTP
pH, DO, Temperature, BOD5 and COD parameters were 8.40, 2.15mg/l, 27oC, 373mg/l and 637.5mg/l respectively. pH was significantly different between influent and effluent (p<0.01), but not in Temperature. The other non-normally distributed parameters were used the Kruskal-Wallis test for Cr, EC, TDS, BOD5, COD and DO were significantly different (p<0.05). EC, TDS and Chromium concentration were recorded higher than standards in the effluent of wastewater treatment plant, and may indicated that the treatment plant is limited to treat these parameters.12, 16
Assessing the lowest chromium bioaccumulation vegetables
The main objective of this research was to identify the least chromium bioaccumulation vegetables in their edible parts only. Chromium bioaccumulation result was recorded highest in root edible and lowest in fruit edible vegetables in both sites (Figure 3‑2). Chromium bioaccumulation in root is sometimes 100-times higher than the shoots and fruits.17 In addition, the highest chromium bioaccumulation was in carrot and lowest in maize (Figure 3‑2). The chromium bioaccumulations capacity in different vegetable edible parts was in the order of roots > stem > leaves > fruits, since there is natural translocation defence of chromium from root to other part of vegetables.18 According the previous study highest chromium concentration was recorded in the cell walls of roots and root parts and the cell wall fraction contained major portion of chromium concentration (83.2%) in roots.19 In general, in this study the chromium bioaccumulation order of vegetables were root edible > leaf edible > fruit edible and as increase the length of edible part from soil part, it could be increased the natural defence and can record lowest chromium bioaccumulation capacities (Figure 3‑2).18
 |
Figure 2: Shows Chromium concentration in vegetables in Edible and Vegetable types respectively Click here to view Figure |
Conclusion and Recommendations
Conclusion
The effect of Sheba leather industry wastes on environment has shown its contribution on the irrigation water, soil and vegetables of Wukro irrigation areas. The efficiency of the leather industry wastewater treatment plant is limited to treats in some parameters, like EC, TDS and Chromium. In the vegetable samples was recorded highest chromium concentration in root edible vegetables, while the lowest chromium bioaccumulation was found in fruit edible vegetables. The lowest chromium bioaccumulation vegetable was in maize and highest was measured in carrot from fruit and root edible vegetables respectively.
Recommendations
- Root and leaf edible vegetables are strictly forbidden to cultivate downstream of leather industries.
- The efficiency of Sheba leather industry wastewater treatment plant is limited in some parameters; it recommended cooperation with government, Universities and other organization to solve the problems.
- Sheba leather industry may contribute pollution to the environment, thus federal and regional environmental protection authorities have to strengthen their regulation.
- Environmental laws have to be strictly enforced by the federal and regional environmental protection authorities to control improper tannery and other industrial waste generation.
- There is no clear environmental standard prepared by Environmental protection Authority of Ethiopia on tannery wastes. Therefore, this standard should be prepared, disseminated and enforced.
- Further researches are recommended, in vegetables chromium bioaccumulations including the soil physico-chemical characterization and properties in Sheba leather industry irrigation areas and related industries.
Acknowledgements
I would like to thank Adigrat University for financial and material supports. Thank you to Sheba leather industry employees (specially, to Mr. G/meskel G/yohans, production department manager for his kind and constructive advises). I need to thank to Ezana Analytical Laboratory, Mekelle, Tigray, Ethiopia, for their help in sample analysis. I thank you Dr. Shivraj Sahai to his constructive comment and editing the paper. Last not least special thanks to my colleague’s Mr. G/Mariam G/her and Mr. Alem Mezgebo, it was good opportunity working with them during field sample collection and sharing of information with statistical data analysis.
Funding
The author(s) received financial support from Adigrat University.
Conflict of Interest
The authors do not have any conflict of interest.
Reference
- Nath, K., S. Saini, and Y.K. Sharma. Chromium in tannery industry effluent and its effect on plant metabolism and growth. Journal of Environmental Biology. 2005; 26(2): p. 197-204.
- Sahu, R., et al. Assessment of drain water receiving effluent from tanneries and its impact on soil and plants with particular emphasis on bioaccumulation of heavy metals. Journal of Environmental Biology. 2007; 28(3): p. 685.
- Misra, V. and S. Pandey. Immobilization of heavy metals in contaminated soil using nonhumus–humus soil and hydroxyapatite. Bulletin of environmental contamination and toxicology. 2005; 74(4): p. 725-731.
- Akinola, M. and T. Ekiyoyo. Accumulation of lead, cadmium and chromium in some plants cultivated along the bank of river Ribila at Odo-nla area of Ikorodu, Lagos state, Nigeria. Journal of Environmental Biology. 2006; 27(3): p. 597-599.
- Aydinalp, C. and S. Marinova. The effects of heavy metals on seed germination and plant growth on alfalfa plant (Medicago sativa). Bulgarian Journal of Agricultural Science. 2009; 15(4): p. 347-350.
- Singh, A.K., P. Misra, and P. Tandon. Phytotoxicity of chromium in paddy(Oryza sativa L.) plants. Journal of Environmental Biology. 2006; 27(2): p. 283-285.
- MEMON, A.R., et al. Heavy metal accumulation and detoxification mechanisms in plants. Turkish Journal of Botany. 2001; 25(3): p. 111-121.
- Biru, A. Assessment of the fertility and pollution status of irrigated vegetable farms around Addis Ababa City Agricultural Office. Final Report. Addis Ababa, Ethiopia. 2002.
- Balkhair, K.S. and M.A. Ashraf. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi journal of biological sciences. 2016; 23(1): p. S32-S44.
- Al Bakheet, S.A., et al. Effect of long-term human exposure to environmental heavy metals on the expression of detoxification and DNA repair genes. Environmental Pollution. 2013; 181: p. 226-232.
- Amabye, T.G. Plant, soil and water pollution due to tannery effluent a case study from Sheb Tannery, PLC, Wukro Tigray, Ethiopia. Science Journal of Analytical Chemistry. 2015; 3(5): p. 47.
- Gan, Y., et al. Multiple factors impact the contents of heavy metals in vegetables in high natural background area of China. Chemosphere. 2017; 184: p. 1388-1395.
- Tesfay, Y., et al. LIVES feed value chain development. Approaches and scalable interventions. 2016.
- Gebrekidan, A., et al. Toxicological assessment of heavy metals accumulated in vegetables and fruits grown in Ginfel river near Sheba Tannery, Tigray, Northern Ethiopia. Ecotoxicology and environmental safety. 2013; 95: p. 171-178.
- Abegunrin, T., et al. Impact of wastewater irrigation on soil physico-chemical properties, growth and water use pattern of two indigenous vegetables in southwest Nigeria. Catena. 2016; 139: p. 167-178.
- Maleki, A., et al. Spatial distribution of heavy metals in soil, water, and vegetables of farms in Sanandaj, Kurdistan, Iran. Journal of Environmental Health Science and Engineering. 2014; 12(1): p. 136.
- Shanker, A.K., et al Chromium toxicity in plants. Environment international. 2005; 31(5): p. 739-753.
- Tiwari, K., et al. Chromium (VI) induced phytotoxicity and oxidative stress in pea (Pisum sativum L.): biochemical changes and translocation of essential nutrients. 2009.
- Caldelas, C., J. Bort, and A. Febrero. Ultrastructure and subcellular distribution of Cr in Iris pseudacorus L. using TEM and X-ray microanalysis. Cell biology and toxicology. 2012; 28(1): p. 57-68.







