Acute Toxicity Bioassay of a Pyrethroid Pesticide Bifenthrin to the Asian stinging Catfish, Heteropneustes Fossilis (Bloch)
1
Department of Zoology,
Sundarban Hazi Desarat College,
South 24 Parganas,
743611,
West Bengal,
India
2
Department of Zoology,
S.B.S. Government College,
Hili,
733126,
West Bengal,
India
3
Office of the Deputy Director of Fisheries (Kolkata Zone),
Government of West Bengal,
9A, Esplanade East, Kolkata,
700 069,
West Bengal,
India
4
Fisheries Ecotoxicology Research Laboratory (Vice-Chancellor’s Research Group), Department of Zoology,
University of Burdwan,
Golapbag, Bardhaman,
713 104,
West Bengal,
India
DOI: http://dx.doi.org/10.12944/CWE.16.1.25
Copy the following to cite this article:
Saha S, Mukherjee D, Dhara K, Saha N. C. Acute Toxicity Bioassay of a Pyrethroid Pesticide Bifenthrin to the Asian stinging Catfish, Heteropneustes Fossilis (Bloch). Curr World Environ 2021;16(1). DOI:http://dx.doi.org/10.12944/CWE.16.1.25
Copy the following to cite this URL:
Saha S, Mukherjee D, Dhara K, Saha N. C. Acute Toxicity Bioassay of a Pyrethroid Pesticide Bifenthrin to the Asian stinging Catfish, Heteropneustes Fossilis (Bloch). Curr World Environ 2021;16(1). Available From : https://bit.ly/37GGW3D
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 11-09-2020 |
|---|---|
| Accepted: | 22-02-2021 |
| Reviewed by: | 
 Dr. J. Sugumaran
Dr. J. Sugumaran
|
| Second Review by: |

 Swarndeep Hundal
Swarndeep Hundal
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Pesticides are being used indiscriminately to maintain a sustainable yield of various crops necessary to support the ever increasing animal population. They constitute a prime component of agricultural runoff which gets intermingled to the adjacent water bodies affecting the life of different non-target organisms (Raina et al., 2009).The contamination of aquatic ecosystem by pesticides is a global problem (Hill, 1985; Sibley and Kaushik, 1991). Human beings are the worst victims of pesticide biomagnifications as they occupy the apex of the food pyramid (Sahai, 1992).
Bifenthrin is a type-I synthetic neopyrethroid having eight stereoisomers with the cis-isomer being the active ingredient (Khan et al., 2013). Pyrethroids act by exerting a time lag in the closing of sodium ion channels present on nerve cells even after an initial entry of sodium ions during the phase of depolarization of action potential (Saha and Kaviraj, 2008; Khan et al., 2013). This culminates in a sustained sodium ion flow. The absence of α-cyano-group assists bifenthrin to bind to the sodium ion channels promoting the generation of after potentials followed by sustained firing of the axon, ineffective to the resting potential (Khan et al., 2013). Bifenthrin is also characterized by reduced environmental degradation and strong insecticidal effects (Mokry and Hoagland, 1989). It is also a popular stomach or contact insecticide and affects cellular ATPase production (Velisek et al., 2009; Roberts and Hutson, 1999). Though there are limited studies on the toxicity of Bifenthrin and its nanoencapsulated form to rainbow trout (Velisek et al., 2009), there is no report of its effects on air breathing fishes. Our study is the first of its kind and future chronic studies may be carried out to elucidate more detailed knowledge on the various aspects of toxicity to air breathing fishes.
The present research has the following objectives:
- To determine the acute toxicity of the pesticide bifenthrin to H. fossilis in order to ascertain its safe permissible levels for the water bodies of our country.
- To determine the behavioural response and the alteration in respiratory rate of H. fossilis as a result of the toxic insult.
Materials and Methods
The Asian stinging catfish, Heteropneustes fossilis (Order: Siluriformes; Family: Heteropneustidae) was used as the test animal in the bioassay having a mean length of 11.7 ± 0.3 cm and a mean weight of 21.60 ± 0.7 g. It was procured from a nearby aquaculture farm followed by acclimatization under experimental ambience for three days prior to their use. During acclimatization the test fish were kept in the rectangular cemented tanks of 1000 litre capacity filled with unchlorinated water (pH 7.20 ± 0.35; temperature 26.53 ± 1.12 °C) for 12 h each (dark and light cycle) (APHA, 2012). The food was supplied to the fish in the form of commercial pellets with 36% crude protein. The acclimatized fish were not fed 24h before the start of the bioassay for maintaining their normal metabolic activity (APHA, 2012).
The analytical grade Bifenthrin [IUPAC name: 2-methylbiphenyl-3-ylmethyl (Z)-(1RS,3RS)-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate] (Brand Name: Marker, marketed by Dhanuka Agritech Limited) was used as the test chemical. It is a third-generation synthetic pesticide belonging to the pyrethroid family (Bansode and Patil, 2016).
Static replacement bioassay with the healthy, disease free fish (irrespective of sex) was conducted in 18l glass aquaria containing 10l unchlorinated water following standard protocols of American Public Health Association(APHA, 2012).The limnological numeric of different water quality criterion for the experiment were:temperature 25.5 ± 0.65 °C, pH 7.4 ± 0.55, free CO2 10.3 ± 0.15 mg/l, DO 5.39 ± 0.23 mg/l, alkalinity 170 ± 9.11 mg/l as CaCO3, hardness 116 ± 4.70 mg/l as CaCO3.The experimental design comprised of four replicates along with a control. Each replicate comprised of twenty fish. Fish were not fed for 24h prior to the experiment.
A preliminary range finding test was conducted to demarcate the concentration range at which mortality of fish may occur. The final test concentrations of bifenthrin chosen to determine the 24, 48, 72 and 96h median lethal concentration (LC50) values were 3.0, 3.5, 4.0, 4.5, 5.0, 5.5 and 6.0 µg/l. During the bioassay, the dead organisms were removed from the aquaria for avoiding any microbial decomposition. The count of dead fish was recorded after every 24h of experiment. Approximately, about 10% of the test water was replaced by newly prepared test water at every 12h interval to maintain a uniform concentration of the pesticide.
Toxicity factors of the test fish to bifenthrin at different time of exposure were assessed by dividing LC50 value at 24h by LC50value at any other exposure time (Ayoola et al. 2011).
The safe level estimation for H. fossiliswas obtained as a product of the 96h LC50 with different application factors (AF) based on Edwards and Brown (Edwards and Brown, 1966), Burdick (Burdick, 1967), Sprague (Sprague, 1971), Committee on Water Quality Criteria (CWQC, 1972), International Joint Commission (IJC, 1977), European Inland Fisheries Advisory Commission (EIFAC, 1983)and Canadian Council of Resources and Environmental Ministry (CCREM, 1971)besides the formula given by Hart et al. (Hart et al., 1948).
The probit program version 1.5 software was used to calculate the LC50(with 95% confidence limit) from mean mortality data of H. fossilisobtained after 24, 48, 72 and 96h of bioassay(US EPA, 1999). Median lethal concentration (LC50) was determined in MS Excel by plotting the test concentrations against the fish mortality within 24h, 48h, 72h and 96h after the bioassay (Finney, 1971). The relation between rate of mortality with exposure time and concentrations was evaluated using correlation analysis (US EPA, 1999; US EPA, 2006; Gomez and Gomez, 1984).The behavioral changes like restlessness, erratic swimming, and mucus secretion in the treated fish were also recorded during the bioassay (Saha et al., 2018; Dasgupta et al., 2010; Saha et al., 2020). Changes in the opercular movements in order to determine respiratory rate of H. fossilis exposed to selected doses of bifenthrin was also noted for the entire experimental period. Opercular movements of the fish per minute for both control and treated sets were counted twice a day during the bioassay and their mean values per dose were plotted graphically.The statistical tool of analysis of variance (ANOVA) given in R-software (R Development Core Team., 2012) followed by multiple mean comparison using Duncan’s Multiple Range Test (DMRT) was applied to the opercular movement data in order to find out significant difference within the average values at different doses of bifenthrin at 24, 48, 72 and 96h time slots.
Results
No test organism died during the acclimatization period. The acute toxicity of bifenthrin (LC1,5,10,15,50,85,90,95,99) with 95% confidence limit to H. fossilis during the exposure period of 24, 48, 72 and 96h are shown in Table 1. The control set showed no mortality of fish during the entire test period.
Table 1: Acute lethal concentration (LC1,5,10,15,50,85,90,95,99) values with 95% confidence limits of bifenthrin to H. fossilis at 24, 48, 72 and 96h (Theoretical spontaneous response rate in control group = 0.000).
|
Points of Lethal Concentration |
Dose values (µg/l)with 95% confidence limits in parentheses |
|||
|
24h |
48h |
72h |
96h |
|
|
LC1 |
1.669 (0.721-2.299) |
1.627 (0.754-2.223) |
1.572 (0.863-2.071) |
1.560 (0.860-2.051) |
|
LC5 |
2.277 (1.270-2.860) |
2.188 (1.263-2.745) |
1.994 (1.261-2.468) |
1.961 (1.239-2.428) |
|
LC10 |
2.687 (1.714-3.220) |
2.562 (1.661-3.077) |
2.263 (1.543-2.712) |
2.216 (1.505-2.658) |
|
LC15 |
3.005 (2.095-3.495) |
2.850 (1.995-3.329) |
2.466 (1.766-2.893) |
2.406 (1.715-2.828) |
|
LC50 |
4.821 (4.361-5.539) |
4.471 (4.034-4.979) |
3.541 (3.078-3.863) |
3.407 (2.933-3.724) |
|
LC85 |
7.734 (6.394-12.464) |
7.014 (5.975-10.165) |
5.085 (4.635-5.968) |
4.824 (4.420-5.568) |
|
LC90 |
8.650 (6.926-15.262) |
7.803 (6.461-12.213) |
5.539 (4.975-6.790) |
5.238 (4.741-6.291) |
|
LC95 |
10.208 (7.785-20.632) |
9.137 (7.241-16.061) |
6.288 (5.496-8.263) |
5.917 (5.225-7.589) |
|
LC99 |
13.929 (9.671-36.406) |
12.286 (8.941-26.927) |
7.977 (6.578-12.029) |
7.438 (6.217-10.880) |
|
Slope ± SE |
5.048±1.174 |
5.299±1.166 |
6.595±1.272 |
6.860±1.325 |
|
Intercept ±SE |
1.550±0.773 |
1.553±0.761 |
1.378±0.795 |
1.347±0.819 |
There was a significant variation (p<0.05) in the mortality of the treated fish exposed to bifenthrin with respect to the control at all the hours of exposure. A significant variation (p<0.05) between rate of mortality of H. fossilis and time slots (24-96h) was recorded for the final selected doses of bifenthrin except 4.0, 4.5 and 5.5 µg/l concentration of the toxicant. The relationship between concentration of bifenthrin and fish mortality at 24 h was, y = 27.75ln(x) + 9.051, R² = 0.925; it was y = 29.37ln(x) + 12.79, R² = 0.887at 48h (Figure 1); at 72 h it was y = 31.30ln(x) + 31.16, R² = 0.912 and at 96 h was y = 30.22ln(x) + 36.05, R² = 0.904 (Figure 2).
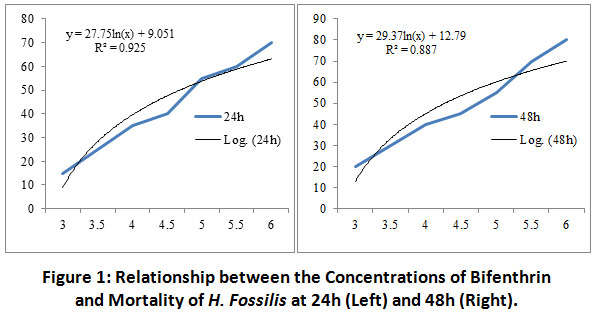 |
Figure 1: Relationship between the Concentrations of Bifenthrin and Mortality of H. Fossilis at 24h (Left) and 48h (Right). Click here to view Figure |
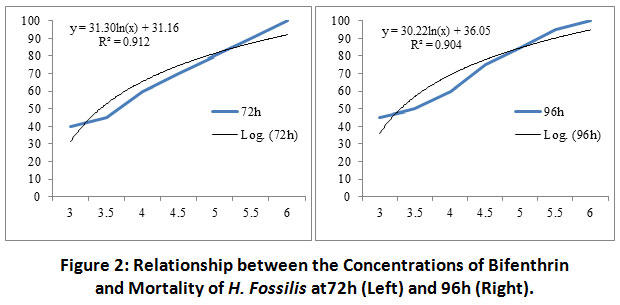 |
Figure 2: Relationship between the Concentrations of Bifenthrin and Mortality of H. Fossilis at72h (Left) and 96h (Right). Click here to view Figure |
The toxicity factors as calculated from median lethal toxicity (LC50) values at different times of exposure are tabulated in Table 2. With the progress of time, the toxicity factor values for the tested fish species to bifenthrin increased gradually.
Table 2: Toxicity factors for H. fossilis exposed to bifenthrin at different time scale (24, 48, 72 and 96h).
|
Exposed time (h) |
Toxicity factor value |
|
24 |
1.000 |
|
48 |
1.078 |
|
72 |
1.361 |
|
96 |
1.415 |
The estimated possible safe level of bifenthrin for the fish is stated in Table 3. In the current experiment, safe level estimated for bifenthrin ranged from 0.0341 – 1.3628µg/l.
Table 3: Safe levels of bifenthrin to H. fossilis at 96hours of exposure according to some International standard methods.
|
Name of the test organism |
96h LC50 value (µg/l) |
Method |
Application factor (AF) |
Safe level (µg/l) |
|
H. fossilis |
3.407 (2.933-3.724) |
Hart et al. (1948)* |
|
0.1154 |
|
Edwards and Brown (1966) |
0.4 |
1.3628 |
||
|
Burdick (1967), Sprague (1971) and EIFAC (1983) |
0.1 |
0.3407 |
||
|
CWQC (1972) |
0.01 |
0.0341 |
||
|
IJC (1977) |
5% of 96h LC50 |
0.1704 |
||
|
CCREM (1991) |
0.05 |
0.1704 |
(*C = 48h LC50 X 0.03/S2, where C= presumable harmless concentration; S = 24h LC50/48h LC50)
The ethological alterations noted in H. fossilis under exposure to various doses of bifenthrin are summarized in Table 4. The intensity of behavioral alterations increased with the increasing doses and progress of time of exposure (Table 4).
Table 4: Impact of Bifenthrin on the behavioral parameters of H. fossilis (R: restlessness; ES: erratic swimming; MS: mucus secretion; NN: not noted due to death; –: none; +: mild; ++: moderate; +++: strong) at various doses during 24-96h time slots.
|
Dose (mg/l)
|
24h |
48h |
72h |
96h |
||||||||
|
Behaviour of H. fossilis |
||||||||||||
|
R |
ES |
MS |
R |
ES |
MS |
R |
ES |
MS |
R |
ES |
MS |
|
|
0.0 |
- |
- |
+ |
- |
- |
+ |
- |
- |
+ |
- |
- |
+ |
|
3.00 |
- |
- |
+ |
- |
- |
+ |
- |
- |
+ |
- |
- |
+ |
|
3.50 |
- |
- |
+ |
- |
- |
+ |
- |
- |
+ |
- |
- |
+ |
|
4.00 |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
4.50 |
+ |
+ |
+ |
++ |
+ |
++ |
++ |
++ |
++ |
++ |
++ |
++ |
|
5.00 |
++ |
++ |
++ |
++ |
++ |
++ |
+++ |
++ |
++ |
++ |
+++ |
++ |
|
5.50 |
++ |
++ |
++ |
++ |
++ |
++ |
+++ |
++ |
+++ |
+++ |
+++ |
+++ |
|
6.00 |
++ |
+++ |
+++ |
NN |
NN |
NN |
NN |
NN |
NN |
NN |
NN |
NN |
The gradual increase in dose of bifenthrin resulted in significant increase (p<0.05) in opercular movement of the fish with respect to the control. On the other hand, opercular movement showed a significant increase (p<0.05) with the advancement of time for all the treated doses.
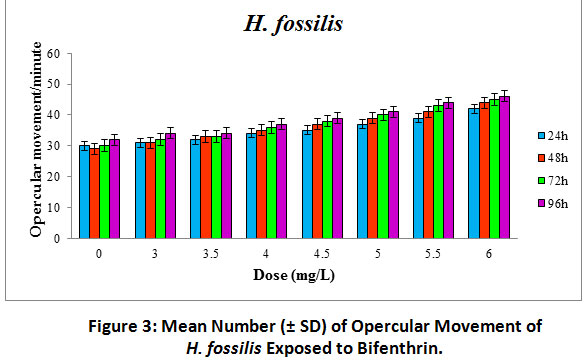 |
Figure 3: Mean Number (± SD) of Opercular Movement of H. fossilis Exposed to Bifenthrin. Click here to view Figure |
Discussion
Pesticides belonging to the group of pyrethroid present a risk for aquatic organisms, though they have low toxicity for aves and mammals (Bradbury and Coats, 1989). The current investigation shows the 96h median lethal concentration of bifenthrin as 3.407μg/l. The present value of 96h LC50 of bifenthrin to the exposed fish (3.407μg/l) is much higher in comparison to rainbow trout (1.47 μg/l), common carp (2.08 μg/l) and tilapia (0.80 μg/l) (Velisek et al., 2009, Liu et al., 2005). This variation may be because of difference in physicochemical parameters of the experimental water, age, size, health and species of fish (having accessory respiratory organ) used in the present study(Farah et al., 2004; Diedrich et al., 2015; Patra et al., 2015). Temperature of the test water may be a key factor in determining the degree of toxicity since lower temperature increases the toxic potential of bifenthrin to fish (Mauck et al, 1976).
Living organisms in response to an adverse surrounding exhibit its defensive nature by means of a vital parameter called tolerance (Enuneku and Ezemonye, 2012). Toxicity factor (TF) is an important index for tolerance assay (Ayoola et al. 2011). In the current investigation, the toxicity factor for the neo pyrethroid, bifenthrin increases in fish with the length of period of exposure (Table 2). This is corroborated by the study of Ayoola et al. (2011). In our present investigation, the estimated possible safe level of bifenthrin to fish showed large variation (0.0341 – 1.3628 µg/l) due to different values of application factors (AFs) according to some International standard methods (Kennega, 1979) (Table 3).
Various changes in the ethological responses are the primary indicators of signs of toxicity to a given xenobiotic. Likewise, our study considered changes in ethology as an important tool to assess bifenthrin toxicity in fish (Table 4). Initially, hyperactivity was noted in the treated fish with respect to the control. With the advancement of time and gradual rise in concentration H. fossilis exhibited the symptoms of stress build up which was manifested as erratic swimming, restlessness, gasping for air, surface adherence etc. Besides, somersaulting pattern in fish also observed at the upper dose limit. Probably, this behaviour was symbolic of an escape reaction from bifenthrin (Saha et al., 2018). The vigorous mucus secretion in H. fossilis may be attributed as an evading mechanism to bifenthrin from entering the body. It is an outcome of stress and irritating effect similar to that of many other neurotoxicants. Some organophosphates also elicit a similar response in fish (Rao et al., 2005; Pandey et al., 2008). At all the exposures the death of fish was characterized by wheezing, repeated turning of the opercula, loss of balance, disruption in buoyant behaviour, enhanced rigor and momentary cessation of ventilation. Restlessness and erratic swimming of treated fish were probably attributed to the adverse impact of bifenthrin on the cerebrospinal nervous system (Velisek et al, 2009).
In fish, the opercular movements are directly related to respiratory rate, which is often the first physiological response to be affected by the presence of toxicant in the aquatic environment (Dube and Hosetti, 2010). In the present investigation, opercular movement in H. fossilis exposed to bifenthrin was found to increase significantly (p<0.05) in response to all doses of the pesticide (Figure 3). Gills are the principal respiratory organs of fish. The energy demand of different metabolic pathways is met up by the gills. Thus, any damage to this organ may culminate in severe respiratory ailments (Magare and Patil, 2000). The mechanism of toxicant uptake through gills probably occurs through simple diffusion (Opperhuizen et al., 1985). In the current investigation, gradual increase in the flapping of operculum of H. fossilis to bifenthrin may be a compensatory mechanism to overcome respiratory distress (Kumar et al., 2015).
Conclusion
This study reveals that bifenthrin is a potent toxicant and it may cause mortality in H. fossilis at very low concentration, even at short period of exposure. The results of the current experimental work may provide some insights on laying out an action plan for management of this pyrethroid including the marking of maximum permissible limit for this xenobiotic before discharging them to the aquatic ecosystem having a myriad of aquatic organisms in it. In future, more studies on the chronic toxicity of bifenthrin to aquatic organisms may open up new vistas in the field of aquatic toxicology.
Acknowledgment
The authors are thankful to the Principal, Barasat Government College and also to the Department of Zoology, University of Burdwan for allowing them to carry out the research work.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- APHA, AWWA, WPCA. 2012. Standard methods for the examination of water and wastewater. 22nd edn. American Public Health Association, Washington, USA.
- Ayoola SO, Kuton MP, Idowu AA, Adelekun AB. 2011. Acute toxicity of Nile tilapia (Oreochromis niloticus) juveniles exposed to aqueous and ethanolic extracts of Ipomoea aquatic leaf. Nature and Science. 9(3): 91-99.
CrossRef - Bansode SB, Patil RD. 2016. Relative toxicity of bifenthrin and carbosulfan to fresh water fish Gara mullya (Sykes). Biolife. 4(2): 482-484. doi:10.17812/blj.2016.4310
- Bradbury SP, Coats JR. 1989. Toxicokinetics and toxicodynamics of pyrethroid insecticides in fish. Environmental Contamination and Toxicology. 8: 373-380.
CrossRef - Burdick GE. 1967. Use of bioassays in determining levels of toxic wastes harmful to aquatic organisms. American Fisheries Society Symposium. 4: 3-12.
- CCREM. 1971. Canadian Water Quality Guidelines; Canadian Council of Resources and Environmental Ministry, Inland Waters Directorate. Environment Canada. Ottawa, ON, Canada.
- Committee on Water Quality Criteria. 1972. A Report of the Committee on Water Quality. Ecological Research Series, EPA-R3-73-003, US Environmental Protection Agency Report, CWQC; Cincinnati, OH, USA.
- Das Gupta R, Chakravorty PP, Kaviraj A. 2010. Studies on Relative Toxicities of Six Insecticides on Epigeic Earthworm, Perionyx excavates. Bulletin of Environmental Contamination and Toxicology 85: 83-86. https://doi.org/10.1007/s00128-010-0038-5.
CrossRef - Diedrich D, Sofield R, Ranville J, Hoff D, Wall D, Brinkman S. 2015. The effects of chronological age and size on toxicity of zinc to juvenile brown trout. Archives of Environmental Contamination and Toxicology. 69(1): 123-131.
CrossRef - Dube PN, Hosetti BB. 2010. Behavior surveillance and oxygen consumption in the freshwater fish Labeo rohita (Hamilton) exposed to sodium cyanide. Biotechnology in Animal Husbandry. 26(1-2): 91-103. https://doi.org/10.2298/BAH1002091D.
CrossRef - Edwards RW, Brown VM. 1966. Pollution and fisheries. In Institute of Sewage Purification, Annual Conference. 1: 49-55.
- EIFAC. 1983. European Inland Fisheries Advisory Commission. Revised Report on fish toxicity testing procedures. EIFAC Technical Paper. No. 24 Revision 1.
- Enuneku AA, Ezemonye LI. 2012. Acute toxicity of cadmium and lead to adult toad Bufo maculates. Asian Journal of Biological and Life Science. 1(3): 238-241.
- Farah MA, Ateeq B, Ali MN, Sabir R, Ahmed W. 2004. Studies on lethal concentrations and toxicity stress of some xenobiotics on aquatic organisms. Chemosphere. 55(2): 257-265. https://doi.org/10.1016/j.chemosphere.2003.10.063.
CrossRef - Finney DJ. 1971. Probit analysis. Cambridge University Press. London.
- Gomez KA, Gomez AA. 1984. Statistical procedures for agricultural research. 2nd edn. John Wiley and Sons. New York, USA.
- Hart WB, Weston RF, Dermann JG. 1948. An apparatus for oxygenating test solution which fish are used as test animals for evaluating toxicity. Transactions of the American Fisheries Society. 75: 288.
CrossRef - Hill IR. 1985. Effects on non target organisms in terrestrial and aquatic environments. In: Lehey J.P. (ed.): The Pyrethroid Insecticides. Taylor & Francis, London. 165-181 pp.
- IJC. 1977. New and Revised Great Lakes Water Quality Objectives. Great Lake Basin. Windsor, Ottawa, Canada, p.1.
- Kenega EF. 1979. Aquatic test organism and methods useful for assessment of chronic toxicity of chemicals, Analyzing the Hazards Evaluation Process. American Fisheries Society. Washington DC, pp. 101.
- Khan AM, Sultana M, Raina R, Dubey N, Dar SA. 2013. Effect of Sub-Acute Toxicity of Bifenthrin on Antioxidant statusand Hematology After its Oral Exposure in Goats. Proceedings of the National Academy of Sciences, India, Section B Biological Science. 83(4): 545-549. https://doi.org/10.1007/s40011-013-0157-y
CrossRef - Kumar M, Kumar P, Devi S. 2015. Toxicity of copper sulphate on behavioural parameter and respiratory surveillance in fresh water catfish, Clarias batrachus (Linn.). International Journal of Fauna and Biological Studies. 2(4): 80-85.
- Liu TL, Wang YS, Yen JH. 2005. Separation of bifenthrin enantiomers by chiral HPLC and determination of their toxicity to aquatic organism. Journal of Food and Drug Analysis. 12: 357-360.
- Magare SR, Patil HT. 2000. Effect of pesticides on oxygen consumption, red blood cell count and metabolites of fish, Puntius ticto. Environment and Ecology 18(4): 891-894.
- Mauck WL, Olson LE, Marking LL. 1976. Toxicity of natural pyrethrins and five pyrethroids to fish. Archives of Environmental Contamination and Toxicology. 4: 18-29.
CrossRef - Mokry LE, Hoagland KD. 1989. Acute toxicities of five synthetic pyrethroid insecticides to Daphnia magna and Ceriodaphnia dubia. Environmental Toxicology and Chemistry. 9: 1045-1051.
CrossRef - Opperhuizen A, Gobas Van Der VEW, Liem FAPC, Van Der Steen JMD. 1985. Relationship between bioconcentration in fish and steric factor of hydrophobic chemicals. Chemosphere. 14(11-12): 1871-1896.
CrossRef - Pandey RK, Singh RN, Das VK. 2008. Effect of temperature and behavioral responses to freshwater catfish Heteropneustes fossilis (Bloch) exposed to dimethoate. Global Journal of Environmental Research. 2(3): 126-132.
- Patra RW, Chapman JC, Lim RP, Gehrke PC, Sunderam RM. 2015. Interactions between water temperature and contaminant toxicity to freshwater fish. Environmental Toxicology and Chemistry. 34(8): 1809-1817.
CrossRef - R Development Core Team. 2012. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3-900051-07-0. Available from: http://www.Rproject.org (accessed on 2019 Oct 8).
- Raina R, Belzer W, Jones K. 2009. Atmospheric concentrations of captan and folpet in the lower Fraser Valley agricultural region of Canada. Air, Soil, Water Research. 2: 41-49.
CrossRef - Rao JV, Begum G, Pallela R, Usman PK, Rao RN. 2005. Changes in behavior and brain acetylcholinesterase activity in mosquito fish Gambusia affinis in relation to sublethal exposure of chlorpyrifos. International Journal of Environmental Research and Public Health. 2(3-4): 478-483.
CrossRef - Roberts T. Hutson D. 1999. Metabolic Pathways of Agrochemicals. Part 2: Insecticides and Fungicides. The Royal Society of Chemistry, Cambridge, United Kingdom. 1180-1384.
- Saha S, Kaviraj A. 2008. Acute toxicity of synthetic pyrethroid cypermethrin to some freshwater organisms. Bulletin of Environmental Contamination and Toxicology. 80: 49-52.https://doi.org/10.1007/s00128-007-9314-4.
CrossRef - Saha S, Mukherjee D, Saha NC. 2018. Evaluation of acute toxicity and behavioral responses of Heteropneustes fossilis (Linn.) exposed to Captan. International Journal of Life Sciences. Volume 6(1): 205-208.
- Saha S, Mukherjee D, Dhara K, Saha NC. 2020. Captan-Induced Toxicity and Behavioural Alterations on Oligochaete Worm, Branchiura sowerbyi. Journal of Aquatic Biology & Fisheries. 8: 37-40.
- Sahai S. 1992. Accumulation and induced histopathological effects of pesticides in the testis of Puntius ticto Teleostei. Journal of Ecobiology. December. 44: 303-308.
- Sibley PK. Kaushik NK. 1991. Toxicity of microencapsulated permethrin to selected nontarget aquatic invertebrates. Archives of Environmental Contamination and Toxicology. 20: 168-176.
CrossRef - Velisek J, Svobodova Z, Piackova V. 2009. Effects of acute exposure to bifenthrin on some haematological, biochemical and histopathological parameters of rainbow trout (Oncorhynchus mykiss). Veterinarni Medicina. 54(3):131-137.
CrossRef - US EPA. 1999. Probit program version 1.5. Ecological Monitoring Research Division, Environmental Monitoring Systems Laboratory, US Environmental Protection Agency. Cincinnati, Ohio. 45168. http:// www.epa.gov/nerleerd/stat2.htm
- US EPA. 2006. Statistical analysis for biological methods. Ecological exposure research division, US EPA. http://www.epa.gov/nerleerd/stat2.htm







