Behavioral Biomarker Responses of Filopaludina bengalensis to Acute Copper Toxicity
Corresponding author Email: pratyushghosh60@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.16.1.23
Copy the following to cite this article:
Ghosh P, Dutta M, Panigrahi A. K. Behavioral Biomarker Responses of Filopaludina bengalensis to Acute Copper Toxicity. Curr World Environ 2021;16(1). DOI:http://dx.doi.org/10.12944/CWE.16.1.23
Copy the following to cite this URL:
Ghosh P, Dutta M, Panigrahi A. K. Behavioral Biomarker Responses of Filopaludina bengalensis to Acute Copper Toxicity. Curr World Environ 2021;16(1). Available From : https://bit.ly/2OiBs8D
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 19-08-2020 |
|---|---|
| Accepted: | 08-03-2021 |
| Reviewed by: | 
 Chuck Chuan
Chuck Chuan
|
| Second Review by: |

 Levent Bat
Levent Bat
|
| Final Approval by: | Dr. Saravanan Pichiah |
Introduction
Indiscriminate water pollution due to urbanization, industrialization and modern agricultural practices alter physical, chemical and biochemical characteristics of water bodies (Indra and Shivaji, 2006). Heavy metal pollution in freshwater ecosystem is of great environmental concern in these days because of bioaccumulative and non-biodegradable nature of the heavy metals (Walker et al., 2006).The natural sources of heavy metals include weathering of rocks and volcanic eruptions, while various industrial activities and mining are their anthropogenic sources (Ali et al., 2019). The concentration of metals in aquatic organisms appear to have been many times greater than the levels that actually exist in the ecosystem (Laws, 2000).Metals become concentrated more at higher trophic levels, presumably due to biomagnification (Wyn et al., 2007). Some heavy metals are essential for life but they could impart toxic effects through bioaccumulation (Gobi et al., 2018). Copper is an essential component of different enzymes but toxic for all forms of life at its excessive level (Gawad, 2018).
A variety of methods have been developed to assess the hazard and toxicity of heavy metals to organisms such as acute toxicity test, chronic toxicity test etc. (Singh and Zahara, 2017). Acute toxicity is the severe or adverse biological effects, which occur within a short period of time after a short term exposure to toxic chemicals (Kopruccu et al., 2006). LC50 or median lethal concentration, a statistically derived concentration, is expected to cause death in 50% of the test species over a given exposure period (Qian et al.,2020). Higher LC50 value indicates less toxic because greater concentrations of the concerned toxicants are required to produce 50% mortality in organisms. Molluscs, specially gastropods and bivalves have been long regarded as promising bioindicators and biomonitoring subjects. They are easily available, easy to grow and extremely tolerant to many pollutants and can also accumulate heavy metals (Kader et al., 2016). Filopaludina bengalensis is a filter feeding benthic gastropod, locally known as ‘Genri’ (Subba Rao and Dey, 1991). They are indiscriminately collected from natural habitats and used as poultry food, cheap proteinaceous food item for rural tribal population in India (Baby et al., 2010).
Generally, the biomarkers are xenobiotically induced measurable physiological, biochemical, behavioral and other alternations in a biological system or samples. Occurrence of biomarkers ranges from subcellular level to whole organism and ecosystem level (Hamza-Chaffai, 2014). Behavior is designed to ensure maximal fitness as it is highly coordinated, structured and predictable sequence of activities rather than a random process (Halappa and David, 2009). Alternations in normal behavioral repertoire can be resulted in reduced fitness and survival of individual organisms, which in turn can elicit undesirable changes in population (Brewer et al., 2001). Behavioral alternations in animals are very sensitive endpoint in ecotoxicology; they can serve as a valuable tool to evaluate effects of exposure to various environmental stressors (Deyashi et al., 2019; Alonso and Camargo, 2011).Copper contamination in freshwater aquatic habitat is very common in Gangetic plains of India. Quest for effective bioindicator species to detect and monitor heavy metal contamination in freshwater systems is the need of the hour. Keeping this in mind, the present study was undertaken with objectives to determine the LC50 value and to evaluate the impact of acute copper intoxication on some behavioral biomarker attributes of Filopaludina bengalensis. This study may serve as a reference for the assessment of the ecotoxicity of copper in many other species. This research could also help to understand the health risks of copper toxicity.
Material and Methods
Snail Collection
The snails Filopaludina bengalensis were collected in the early morning from an unpolluted fish culture pond located near Chandannagar, Hooghly, India (22°87'N and 88°38'E) during June, 2019(Fig. 1). A total 1010 Filopaludina bengalensis were collected by handpicking. They were then transported to laboratory. The shell length of each snail was measured using the divider and meter ruler. Their wet weight was also taken. The specimen selected for the experiment had an average 2.7 cm (range 2.2 to 2.91 cm) shell length and average 2.03 g wet weight (range 1.31 to 2.47 g).They were acclimatized in 5 L glass aquaria for 7 days to laboratory regimes before experimentation. During this acclimatization period, they were fed with blanched lettuce, papaya leaves and libitum plant leaves.
 |
Figure 1: Map Showing the Location of the Snail Collection Site, Near Chandannagar, West Bengal, India. Click here to view Figure |
Bioassay
In order to determine the concentration of heavy metals to be used in the actual experiment, a rough range finding test was carried out for a span of 96 h. The standard stock solutions (100 mg/L) were prepared with deionized water from reagent analytical grade metallic salts of copper. Copper sulfate pentahydrate (CuSO4, 5H2O) was used. Graded series of concentrations were prepared from stock solutions based on APHA (1998) guidelines. Six test concentrations – (0.5, 0.75, 1.0, 1.25, 1.5 and 2.0 ppm) were used for the determination of the LC50 (median lethal concentration) value. The whole experiment was performed at room temperature 27-30°C with photoperiod ~13 h light and ~11 h darkness. Physicochemical parameters of the diluent medium pH, TDS and temperature were determined using portable meters and alkalinity and DO were measured following standard methods of APHA (2012). The mean value and the standard deviation of the parameters for water quality were: Alkalinity: 118.7 ± 15.6 mg/L, DO: 6.93 ± 0.21 mg/L, TDS: 663.5 ± 42.8 mg/L, pH: 7.2 ± 0.2.
Acute toxicity experiment of Cu was performed for 96h period using adult Filopaludina bengalensis. Group of 20 adult snails were placed in 5 L glass aquaria containing appropriate solution. There was a control group with only dechlorinated tap water. The rest of the six groups were exposed to series of metallic salt solution. There were 3 replicates of both control and heavy metal treated groups. A total of 20 snails were used per treatment/concentration including control. Mortality was recorded at the end of 24, 48, 72 and 96 hours of treatment during the toxicity test. The dead snails were removed quickly.
The inability of the animal to respond normally to gentle physical stimulation on foot with blunt needle was used as the criteria for determination of death/mortality. Further confirmation of death was done by placing the animal on glass Petridis for few minutes. If it did not move, it was considered dead. Snails were not fed during the toxicity test.
Study of the Behavioral Responses
Four experimental sets : Set-A (exposed up to 24 h), Set-B (exposed up to 48 h), Set-C (exposed up to 72 h) and Set-D (exposed up to 96 h) each containing 20 snails were exposed to 0.586 ppm (96 h LC50) copper solution. Equal number of individuals exposed to dechlorinated tap water was maintained as control set. Three replicates were set up for each of the sets (both treatment groups and control). Behavioral responses of Filopaludina bengalensis of both the treated and control groups of different exposure period (24 hours, 48 hours, 72 hours, and 96 hours) were recorded. Three types of behavioral responses were observed and recorded here- i) Aggregation and clumping tendency ii) Mucus secretion and iii) Foot protrusion and foot movement. These behavioral responses were documented at 6 h interval in both control and exposed groups. All the results are expressed in percentage.
Statistical Analysis
Finney's Probit Analysis LC50 determination method was used to measure LC50 values, 95% confidence limits, slopes and intercepts of 24 hours, 48 hours, 72 hours, and 96 hours (Finney 1971). As none of the three behavioral measures did not confirm to normality assumption (Kolmogorov – Smirnov test, P < 0.05), for multiple comparison Kruskal-Wallis test and Mann-Whitney U test was done with SPSS version18 software. The results obtained from the behavioral study were represented as mean ± SE (Standard Error).
Results
Acute toxicity of copper revealed that mortality is proportional to the concentration of heavy metal directly. No mortality was evidenced in control. All snails survived in the control throughout the experiment period.
After 24, 48, 72, and 96 hours of exposure, the median lethal concentration (LC50) of copper to Filopaludina bengalensis were 0.852, 0.753, 0.729 and 0.586 ppm respectively. In line with an increase in the span of exposure from 24 to 96 hours, a concomitant decrease in slope function has been noticed. It was also evident that there was an increase in mortality with duration of intoxication. All the median lethal concentration (LC50) values (with 95% confidence limits) at different exposure period (24h, 48h, 72h and 96h) are presented in table 1.
Table 1: LC50 Values of the Copper to Filopaludina Bengalensis at Different Exposure Period.
|
Exposure period (h) |
LC50 (ppm) |
95% confidence limit |
Slope ± SE |
Intercept ± SE |
|
24 |
0.852 |
0.231-1.261 |
1.07 ± 0.207 |
-0.912 ± 0.234 |
|
48 |
0.753 |
0.127-1.11 |
1.213 ± 0.218 |
-0.914 ± 0.238 |
|
72 |
0.729 |
0.226-1.036 |
1.337 ± 0.230 |
-0.974 ± 0.244 |
|
96 |
0.586 |
0.123-0.843 |
1.821 ± 0.298 |
-1.068 ± 0.273 |
Percentage of organisms showing clumping or aggregation response in control sets was ranged between 77.91 ± 1.68% to 82.08 ± 1.29% against different span of exposure, with the advancement of exposure period, gradual deaggregation was observed in snails of treated sets. At 96 h exposure in treated sets aggregation in percent declined to 25 ± 1.23% (Fig.2). As shown by the results of the Mann-Whitney U test, the aggregation response in control sets was significantly (P <0.01) different from the response of snails in treated sets at different span of exposure. Kruskal – Wallis test revealed that there was statistically significant difference in aggregation response at different span of exposure in treated groups [χ2 (3) = 41.14, P <0.01], but difference in response was not significant in case of control groups.
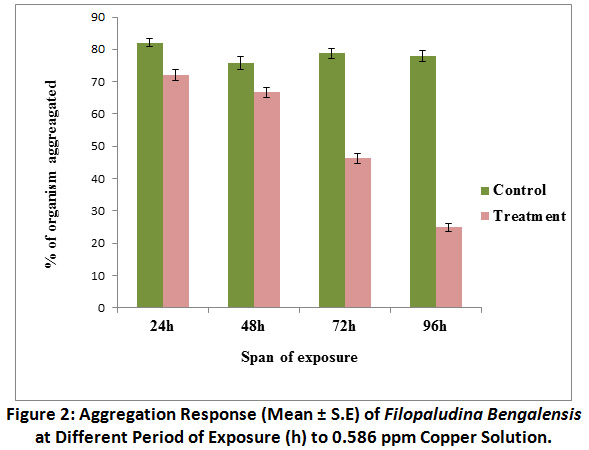 |
Figure 2: Aggregation Response (Mean ± S.E) of Filopaludina Bengalensis at Different Period of Exposure (h) to 0.586 ppm Copper Solution. Click here to view Figure |
Normal low level transparent mucus secretion in few snails was observed in control sets throughout the span of this study. But in treated groups profuse thick whitish mucus secretion was observed. In treated sets, most of the snails started heavy mucus secretion after 48 hours of exposure. At 96 hours of exposure it reached at maximum level (85.4 ± 0.96%). In contrast, <10% organisms of control, secreted heavy visually detectable mucus (Fig. 3). Here again mucus secretion response (expressed in %) was significantly different from that of control groups. A significant increase [χ2 (3) = 5.71, P < 0.01] in mucus secretion (%) was evident in treated sets with the increasing span of exposure. Whereas, response was quite indifferent in control sets [χ 2 (3) = 5.71, P = 0.127) irrespective of exposure tenure.
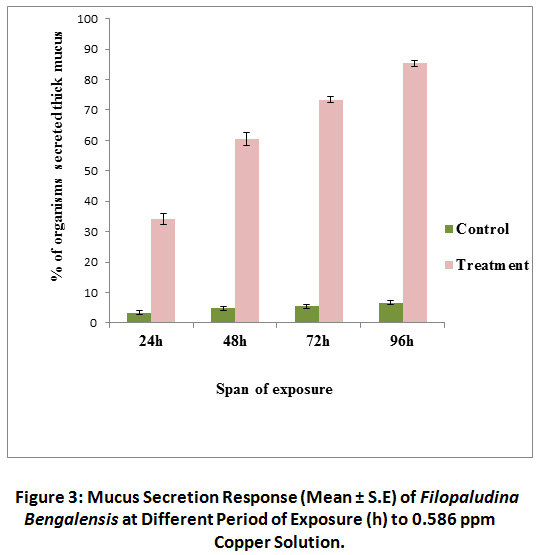 |
Figure 3: Mucus Secretion Response (Mean ± S.E) of Filopaludina Bengalensis at Different Period of Exposure (h) to 0.586 ppm Click here to view Figure |
Copper Solution.
Foot protrusion and movement was inhibited in treated groups. With the increase in span of exposure, concomitant decrease in foot protrusion and movement response was observed (as 85.83 ± 1.35%) at 48 h exposure in control, which was a bit higher than what observed at 24 h exposure. Lowest foot protrusion and movement response (15.41 ± 1.29%) was recorded at 96 h in treated groups (Fig.4).Foot protrusion and movement in the treated groups were significantly different (P<0.01) from control. Results of Kruskal – Wallis test indicated that there was no difference in response in control groups throughout the experimental period [χ 2 (3) = 5.801, P = 0.122). But span of exposure was liable for significant difference in foot protrusion and response in treated sets [χ 2 (3) = 41.18, P <0.01].
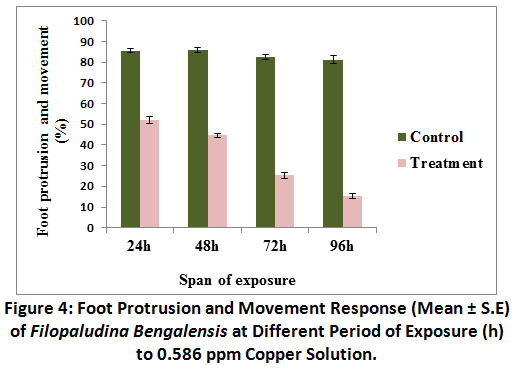 |
Figure 4: Foot Protrusion and Movement Response (Mean ± S.E) of Filopaludina Bengalensis at Different Period of Exposure (h) to 0.586 ppm Copper Solution. Click here to view Figure |
Discussion
The acute toxicity of copper in Filopaludina bengalensis has been reported in a few studies. Direct comparisons of the toxicity values obtained in this study with those in the literature is cumbersome, since they differed between closely related species and also between the same species (Okocha and Adedeji, 2011), primarily due to differences in physicochemical characteristics of the test water and the age of the tested organisms (Wang et al., 2010). The present study reveals that rate of mortality increased as concentration of copper increased and mortality rate rises as the period of exposure increased. These findings also indicate the impact of copper was dependent on dose and exposure time. Kamble and Kamble (2012) showed that 96h-LC50 of copper to Bellamya bengalensis was 0.56 ppm which was quite close to the findings of present investigation. In comparison to other gastropods, the 96 h LC50 value of Copper in Filopaludina bengalensis as observed in the present study is shown in Table 2.
Table 2: Comparison of the 96h LC50 (ppm) of Copper of F. Bengalensis with some other Gastropods.
|
Gastropod Species |
LC50 (ppm) |
References |
|
Melanoides tuberculata |
0.14 |
Shuhaimi – Othman et al. (2012) |
|
Potamopyrgus jenkinsi |
0.08 |
Watton and Hawkes (1984) |
|
Pomacea paludosa |
0.14 |
Rogevich et al. (2008) |
|
Biomphalaria glabrata |
0.18 |
de Oliveira-Filho et al.(2004) |
|
Bellamya bengalensis |
0.56 |
Kamble and Kamble (2012) |
|
Viviparus bengalensis |
0.39 (at 20.3ËšC) |
Gupta et al.(1981) |
|
Filopaludina bengalensis |
0.586 |
Present study |
In this study, it was observed that the behavioral responses of the snails of the treated sets at 24 h, 48 h, 72 h and 96 h were quite different from each other and also different from the responses of the snails in control. The alternations in the behavioral patterns in aquatic organisms are the most sensitive indicators of possible toxic effects of intoxication (Tiwari et al., 2011). In general, intraspecific aggregation in animals ensues due to variety of reasons like food, shelter, parental care, protection etc. (Salma and Thomson 2018; Deyashi et al., 2019).Here, the aggregation response observed in different sets is thought to be a defensive technique to withstand the impact of the metal stressor (Deyashi et al., 2019). Quite similar aggregation response in the same species due to acute exposure of different environmental stressors reported also in some other studies (Bhunia, 2015; Dhara, 2014). This kind of behavioral response as observed in this study was in concordance with the study of Dhara et al. (2017) on exposure of Cadmium on Lymnea acuminata, another related gastropod species.
Filopaludina bengalensis has evolved a well-developed muscular foot which protrudes occasionally through the opercular aperture to fulfill diverse purposes like locomotion, food procurement, reproduction etc. Reduction in foot protrusion and movement as observed in this study was also observed in some other studies. Exposure to copper hydroxide has shown to have an effect on movement of Leidyulla florida (Capinera and Dickens, 2016). Moreover, withdrawal of foot inside the shell can help them to tightly close their operculum (Kamble and Kamble, 2014).
When creeping on substratum, snails naturally secrete watery thin mucus by foot. In the aquatic snail Filopaludina bengalensis, such mucus secretion is also observed. Intoxication of metals caused increased quantity of mucus secretion in gastropods (Yasmeen and Begum, 2015). One of the early responses shown by various gastropods to environmental stressors is increased mucus secretion (Triebskorn et al., 1998). Thick and large amount of mucus was secreted by the foot, while gills secreted somewhat more whitish mucus that comes out of the mouth and in treated sets, its quantity also increased as time went on (Deshmane, 2012). It may be to inhibit metal absorptions into the organism (Lobel, 1981). Mucus acts as a physicochemical barrier of animal against environmental toxins. Extruded mucus forms a kind of protective barrier that helps to keep the epithelia of skin or digestive tract from the direct contact of toxin (Ebenso, 2004; Ebenso et al., 2005). A substantial increase in mucus secretion was observed in Perna viridis upon treatment with 0.5 mg/L copper by Sze and Lee (1995). Heavy mucus secretion in Viviparus bengalensis was observed by Muley and Mane (1988) upon exposure to mercuric salt. Kamble and Kamble (2014) also recorded profuse mucus secretion, reduced foot and tentacular movement as a response to intoxication of copper sulphate. Mollusc also faced the higher risk of dehydration as extruded mucus having higher water content. They lose sufficient energy also, which may leave them motionless (Cottrell et al., 1993; Ebenso, 2004).
An organism's behavior is the final, integrated outcome of various physiological and biochemical processes. Therefore behavioral changes could represent higher organization level of biomarker. A behavioral parameter is more comprehensive and informative than a biochemical or physiological parameter (Walker et al., 2006). The behavioral repertoire in gastropod is limited compared to vertebrates; yet extensive and variable enough to be applied in ecotoxicological studies. The mortality percentage and the biomarker responses observed in this study in Filopaludina bengalensis may be used as indicative parameters for assessing the toxicity of copper in aquatic ecosystems (Doving, 1992).
Conclusion
This study revealed that acute exposure to copper has a major impact on aggregation, foot protrusion response, and secretion of mucus in Filopaludina bengalensis. As the exposure time increased, further deaggregation, inhibition of foot movement and strong mucus secretion were observed. This present study also indicates that the changes in behavioral attributes caused by copper in Filopaludina bengalensis at the individual or population level may be used as an effective biomarker for monitoring and management of aquatic heavy metal pollution.
Acknowledgements
The first author is grateful to the Principal, Chandernagore College and all the faculty members of Department of Zoology, Chandernagore College for their support in carrying out this research work.
References
- Ali H, Khan E, Ilahi I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem.2019; 2019: 1–14.
CrossRef - Alonso Á, Camargo J.A. Subchronic toxic effects of fluoride ion on the survival and behaviour of the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Arch Environ Contam Toxicol.2011; 60(3): 511–517.
CrossRef - APHA. Standard Methods for the Examination of Water and Wastewater. 20. Washington DC, USA: American Public Health Association, American Water Works Association and Water Environment Federation; 1998.
- APHA. Standard Methods for the Examination of Water and Wastewater. 22. Washington DC, USA: American Public Health Association, American Water Works Association and Water Environment Federation; 2012.
- Baby R.L, Hasan I, Kabir K.A, Naser M.N. Nutrient analysis of some commercially important molluscs of Bangladesh. J. Sci. Res. 2010; 2(2): 390-396.
CrossRef - Bhunia N.S. Studies on toxicicological responses of Bellamya bengalensis to detergent. Ph.D. Thesis, University of Calcutta, Kolkata, India. 2015.
- Brewer S.K, Little E.E, DeLonay A.J, Beauvias S.L, Jones S.B, Ellersieck M.R. Behavioral dysfunctions correlate to altered physiology in Rainbow Trout (Oncorynchus mykiss) exposed to cholinesterase-inhibiting chemicals. Arch Environ Contam Toxicol. 2001; 40(1): 70-76.
CrossRef - Capinera J.L, Dickens K. Some effects of copper-based fungicides on plant-feeding terrestrial molluscs: A role for repellents in mollusk management. Crop Prot.2016; 83:76-82.
CrossRef - Cottrell J.M, Henderson I.F, Pickett J.A, Wright D.J. Evidence for glycosaminolycans as a major component of trial mucus from the terrestrial slug Arion ater L. Comp Biochem Physiol. 1993; 104(3): 455-468.
CrossRef - de Oliveira-Filho E.C, Lopes R.M, Paumgartten F.J.R. Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere. 2004; 56(4):369–374.
CrossRef - Deshmane J. Behavioral Responses of Freshwater Snail, Viviparus bengalensis to Plant Toxin in Fruits of Acacia sinuate. Biol Forum. 2012; 4(1): 18-22.
- Deyashi M, Misra K.K, Chakraborty S.B. Evaluation of the acute toxicity of mahua oil cake aqueous extract and its effect on the behavioral responses of the freshwater grapsid crab, Varuna litterata (Fabricius, 1798). Environ Sci Pollut Res. 2019; 26: 15631–15640.
CrossRef - Dhara K, Saha N.C, Maiti A.K. Studies on acute and chronic toxicity of cadmium to freshwater snail Lymnaea acuminata (Lamarck) with special reference to behavioral and hematological changes. Environ Sci Pollut Res. 2017;24:27326-27333.
CrossRef - Dhara K.Hazardous impact of fly ash and some of its ingradients on fish, fish food organisms and aquatic ecosystem. Ph.D. Thesis, University of Kalyani, Kalyani, India. 2014.
- Doving K.B. Assessment of animal behaviour as method to indicate environmental toxicity. Comp Biochem Physiol. 1992; 100: 247–252.
CrossRef - Ebenso I.E, Ita B, Umoren E.P, Ita M, Binang W, Edet G, Izah M, Udo I.O, Ibanga G, Ukpong E.E. Effect of Carbamate Molluscicide on African Giant Land Snail Limicolaria aurora. J. Appl. Sci. Environ. Mgt. 2005; 9 (1):99–102.
- Ebenso I.E. Molluscicidal effects of neem Azadirachta indica extract on edible tropical land snails. Pest Manag. Sci. 2004; 60(2): 178–182.
CrossRef - Finney D.J. Probit Analysis. 3. London: Cambridge University Press;1971.
- Gawad S.S.A. Acute toxicity of some heavy metals to the fresh water snail, Theodoxus niloticus (Reeve, 1856). Egypt. J. Aquat. Res. 2018; 44: 83–87.
CrossRef - Gobi N, Vaseeharan B, Rekha R, Vijayakumar S, Faggio C. Bioaccumulation, cytotoxicity, and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2018; 162: 147–159.
CrossRef - Google.(n.d). https://www.google.co.in/maps (retrieved on 10 January, 2021).
- Gupta P.K, Khangarot B.S, Durve V.S. The temperature dependence of the acute toxicity of copper to a freshwater pond snail Viviparus bengalensis L. Hydrobiologia. 1981; 83: 461-464.
CrossRef - Halappa R, David M. Behavioural Responses of the Freshwater Fish, Cyprinus carpio (Linnaeus) Following Sublethal Exposure to Chlorpyrifos. Turk. J Fish. Aquat. Sc.. 2009;9: 233-238.
- Hamza-Chaffai A. Usefulness of Bioindicators and Biomarkers in Pollution Biomonitoring. Int J Biotechnol Wellness Ind. 2014; 3(1): 19-26.
CrossRef - Indra V, Sivaji S. Metals and organic compounds of sewage and sludges. J.Environ.Biol. 2006; 27: 723-725.
- Kader A.A, Osman G.Y, Mohamed A.H, Gharieb M.M, Ismail N.M.M, Abdel-motleb A. Bioaccumulation of heavy metals in freshwater snails in relation to lining of water courses in Egypt. J. Biosci. Appl. Res. 2016;2 (8): 561–573.
CrossRef - Kamble N.A, Kamble S.B. Reactivity of digestive mucins in freshwater snail Bellamya bengalensis (L.) against copper sulphate induction. Asian.J.Biol.Life Sci. 2012;1(3): 208-212.
- Kamble S.B, Kamble N.A. Behavioural changes in freshwater snail Bellamya bengalensis due to acute toxicity of copper sulphate and Acacia sinuata. Int J Sci Environ Technol. 2014;3: 1090–1104.
- Kopruccu S.S, Kopruccu K, Ural M.S. Acute toxicity of the synthetic pyrethroid deltamehtrin to fingerling European catfish, Silurus glanis L. Bull. Environ Contam Toxicol. 2006; 76: 59-65.
CrossRef - Laws E. Aquatic Pollution- an introductory text.3. New York, U.S.A: John Wiley and Sons;2000.
- Lobel P. Zinc in mussels from an iron pipe. Mar. Pollut. Bull. 1981; 12: 410-411.
CrossRef - Muley D.V, Mane U.H. Seasonal variation in the toxicity of folithion and ledaycid to a freshwater gastropod Viviparus bengalensis (lam) from Godavari River. M.S. India Ad. Biosci. 1988; 1 (7): 37-46.
- Okocha R.C, Adedeji O.B. Overview of cadmium toxicity in fish. J Appl Sci Res. 2011; 7(7): 1195–1207.
- Qian Y, Huang J, Liu X, Liu T, Xue G, Gao P, Zhou X, Zhang Y, Chen J. Rapid oxidation of histamine H2-receptor antagonists by peroxymonosulfate during water treatment: Kinetics, products, and toxicity evaluation. Water Res. 2020; 185:116278.
CrossRef - Rogevich E.C, Hoang T.C, Rand G.M. The effects of water quality and ageon the acute toxicity of copper to the Florida apple snail, Pomacea paludosa. Arch Environ Contam Toxicol.2008; 54(4):690-696.
CrossRef - Salma U, Thomson M. Social aggregation of the marine isopod Cirolana harfordi does not rely on the availability of light-reducing shelters. Physiol Entomol. 2018; 43:60–68.
CrossRef - Shuhaimi–Othman M, Nur–Amalina R, Nadzifah Y. Toxicity of metals to a freshwater snail, Melanoides tuberculata. The Scientific Journal. 2012; 1: 1-10.
CrossRef - Singh A, Zahara K. LC50 assessment of cypermethrin in Heteropneustes fossilis: Probit Analysis. Int. J. Fish. Aquat. Stud. 2017; 5 (5): 126-130.
- Subba Rao N.V, Dey A. Molluscs in aquaculture. In: Jairajpuri M.S. (eds). Snails, Flukes and Man. Calcutta: Zoological Survey of India; 1991: pp. 85-92.
- Sze P.W.C, Lee S.Y. The potential role of mucus in the depuration of copper from the mussels Perna viridis (L.) and Septifer virgatus (Wiegmann).Mar. Pollut. Bull.1995; 31: 390-393.
CrossRef - Tiwari M, Nagpure N.S, Saksena D.N, Kumar R, Singh S.P, Kushwaha B, Lakra W.S. Evaluation of acute toxicity levels and ethological responses under heavy metal cadmium exposure in freshwater teleost, Channa punctata (Bloch). Int. J. Aquat. Sci. 2011; 2: 36-47.
- Triebskorn R, Christensen K, Heim I. Effect of orally and dermally applied metaldehyde on mucous cells of slugs Deroceras reticulatum depending on temperature and duration of exposure. J. Mollus Stud. 1998; 64: 467-487.
CrossRef - Walker C.H, Hopkin S.P, Silby R.M, Peakall D.B. Principles of Ecotoxicology. 3. Boca Raton, Fla, USA: CRC Press;2006.
- Wang N, Ingersoll C.G, Ivey C.D, Hardesty D.K, May T.W, Augspurger T, Roberts A.D, van Genderen E, Barnhart M.C. Sensitivity of early life stages of freshwater mussels (Unionidae) to acute and chronic toxicity of lead, cadmium, and zinc in water. Environ Toxicol Chem. 2010; 29(9): 2053–2063.
CrossRef - Watton A.J, Hawkes H.A. The acute toxicity of ammonia and copper to the gastropod Potamopyrgus jenkinsi (Smith). Environ. Pollut. Ser. A. 1984; 36(1):17–29.
CrossRef - Wyn B, Sweetman J.N, Leavill P.R. Historical metal concentrations in lacustrine food webs revealed using fossil Ephippia from Daphnia. Ecol Appl. 2007; 17(3): 754-764.
CrossRef - Yasmeen S, Begum S.M. Effect of mercury on behaviour of the freshwater bivalves L. Marginalis in different time hours. Int. Sci J. 2015; 2(1): 27-32.







