Enhanced Removal of Organic Pollutants and Reactive Dye in Polyethersulfone Submerged Membrane Bioreactor (PES-SMBR) for Textile Wastewater
1
Department of Chemistry,
Amolakchand Mahavidyalaya,
Yavatmal,
Maharashtra,
India
2
School of Environmental and Earth Sciences,
Kavayitri Bahinabai Chaudhari North Maharashtra University,
Jalgaon,
Maharashtra,
India
DOI: http://dx.doi.org/10.12944/CWE.16.1.33
Copy the following to cite this article:
Chavan T. P, More G. B, Thorat S. R. Enhanced Removal of Organic Pollutants and Reactive Dye in Polyethersulfone Submerged Membrane Bioreactor (PES-SMBR) for Textile Wastewater. Curr World Environ 2021;16(1). DOI:http://dx.doi.org/10.12944/CWE.16.1.33
Copy the following to cite this URL:
Chavan T. P, More G. B, Thorat S. R. Enhanced Removal of Organic Pollutants and Reactive Dye in Polyethersulfone Submerged Membrane Bioreactor (PES-SMBR) for Textile Wastewater. Curr World Environ 2021;16(1). Available From : https://bit.ly/3apX0s8
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2020-08-17 |
|---|---|
| Accepted: | 2021-02-16 |
| Reviewed by: | 
 Akshay Modi
Akshay Modi
|
| Second Review by: |

 Gangadhar Andaluri
Gangadhar Andaluri
|
| Final Approval by: | Dr. Lam Sze Mun |
Introduction
Textile is one of the major industrial sectors in the world which requires huge amounts of consumables for different processes such as dyeing, washing, sizing, finishing and rinsing that consists of many types of chemical reagents include i.e. polyurethane, polyamide, phosphates, softening agent, stiffening agents and chelating agents 1. During textile processing, a large quantity of textile wastewater has been produced. The wastewater generated from textile processing is highly concentrated and it includes huge amounts of ammonia, toxic elements, organic matter and non-biodegradable substances 2–4. These pollutants create serious effects on the environment through a prolonged period, hence appropriate treatment is essential to eliminate such highly toxic pollutants from textile wastewater to decrease the impacts of hazardous chemicals on the environment.
There are various methods available for the treatment of textile wastewater, amongst them physicochemical and biological methods are mostly utilized 5,6. In the physicochemical techniques, various methods are utilized for the treatment of textile wastewater. Some of them are oxidation, ion exchange, adsorption, coagulation, etc. 7,8. Furthermore, in the adsorption, ion exchange and coagulation process, the organic pollutants have releases from one stage to another without any removal. The efficient removal of organic pollutants has been done by electrochemical oxidation, ozonation, Fenton oxidation and photocatalytic oxidation. These processes require high costs and hence, these are not implemented by the industry. On the other hand, the biological processes are the most frequently and cost-effective replacement for the treatment of textile wastewater 9. In contradiction of physicochemical processes, the biological processes are eco-friendly techniques as a result of the total removal of pollutants by preventing the generation of secondary pollutants as well as low maintenance 5,10.
Membrane technology is a combination of an advanced activated sludge process in which the wastewater treatment has been done by the microfiltration and ultrafiltration membranes in the presence of active biomass 11. bioreactor (MBR) technology is vowing to recover the quality of wastewater as well as it will be important to enhance the sustainability of water since MBR gives a boost to the reusability of water and its access option for decentralized treatment. The main benefit of MBR technology is total solids removal, high ability of biodegradation of dyes, nitrogen, carbon and organic pollutants, because of the small pore size of the membrane 12. The typical membrane with having a pore size of 0.01 to 0.1μm can reject the bacteria outside from the wastewater. Therefore, membrane technology is the better choice to eradicate about 98% of organic compounds, bacteria, non-biodegradable substances and dyestuffs as well as it can be a superior option in the replacement of conventional wastewater treatment which boosts industries to make a new invention in membrane technology 13.
After going through the elaborative literature study, an attempt was made in this work to study the performance of commercially available polyethersulfone (PES) membrane for the treatment of textile wastewater in a pilot-scale SMBR model. The purpose of this study is to obtain the ideal operational conditions to boost the removal performance of COD, BOD, NO3-N, TSS, TP, turbidity, conductivity and colour. The performance of polyethersulfone (PES) membrane was conducted in the different HRT and OLR. This work may consider as a preliminary stage before applying membrane technology in the textile wastewater treatment. In addition, the findings of this work can be a reference point for the new researchers who are working on the pilot-scale SMBR model.
Methodology
Selection of Membrane
A thin-film hollow fibre ultrafiltration microporous polyethersulfone (PES) membrane having a mean pore size of 0.02 μm diameter utilized in this work were purchased from Aquaplus Water Purifiers Pvt. Ltd, Pune, India. The surface area of the membrane is 0.038 m2 with an inner and outer diameter of 0.6 and 0.8 mm. In addition, the purchased membrane was characterized by the FESEM and contact angle measurement. Table 1 shows the specification of polyethersulfone (PES) membrane.
Table 1: Membrane Specifications.
|
Technical data |
Values |
|
Manufacturer |
Aquaplus Water Purifiers Pvt. Ltd, Pune |
|
Membrane material |
Polyethersulfone |
|
Geometry |
Hollow fibre |
|
Filtration |
Ultrafiltration |
|
Outer diameter |
0.8 mm |
|
Inner diameter |
0.6 mm |
|
Pore size |
0.02 μm |
|
Surface area |
0.038 m2 |
|
Contact angle |
62.9° |
Characterization of Membrane
FESEM with EDAX
The outer surface and cross-section morphology of the polyethersulfone (PES) membrane was characterized with the help of a FESEM (Model: S-4800, Hitachi, Japan). The sample of polyethersulfone (PES) membrane was deep in liquid nitrogen for getting an accurate sample piece, followed by coating of membrane with gold with the help of ion sputter (Model: E1010, Hitachi, Japan). Additionally, the elemental composition of polyethersulfone (PES) membrane was attained from EDAX (Hitachi, E1010) at Sophisticated Analytical Instrumentation Facility (SAIF), Kavayitri Bahinabai Chaudhari North Maharashtra University, Jalgaon, Maharashtra, India.
Contact Angle Measurement
The contact angle is an important parameter in the identification of hydrophilicity 14. The surface properties and hydrophilicity of the polyethersulfone (PES) membrane were measured by using Rame-Hart Instruments (Model 21 AC), New Jersey, USA with the setup of image-processing software via sessile drop technique. An average of 10 μL each droplet was deposited on the membrane surface and the values of contact angle were taken from six different directions to report the average contact angle.
Synthetic Dye Solution
In this study, the colour removal performance of the SMBR model was conducted by using anionic sulphonate reactive azo dye i.e. Everzol Black as a colour sample. Figure 1 shows the chemical structure of Everzol black. The stock solution of Everzol Black was prepared by dissolving 2 gm of Everzol Black dye in 2000 ml of distilled water. Thereafter, the solution was completely dissolved by heating at 90 °C for 1 hr. The heated solutions were cool down in the water and from this, 1000 ppm of Everzol Black stock solution and 200 ppm of Everzol Black working solution were prepared. The working solution was randomly added to the SMBR model during the entire operation and the removal efficiency was measured by comparing the concentration of influent and permeate solutions. Characteristics of Everzol Black are presented in Table 2.
 |
Figure 1: Chemical Structure of Everzol Black. Click here to view Figure |
Table 2: Characteristics of Everzol Black.
|
Characteristics |
Everzol Black |
|
Maximum absorbance wavelength |
436 nm |
|
Molecular weight (g/mol) |
991.82 |
|
Empirical formula |
C26H21N5Na4O19S6 |
|
pH |
9.92 |
|
Conductivity (µS/cm) |
2496 |
Characteristics of Textile Wastewater
The textile wastewater sample was collected from the rinsing bath of M/s. SMS WALUJ CETP Private Limited, Aurangabad, M.S., India. The characteristics of textile wastewater are given in Table 3.
Table 3: Textile Wastewater Characteristics.
|
Parameters |
Values |
|
COD (mg/L) |
997.16 |
|
BOD (mg/L) |
421.47 |
|
NO3-N (mg/L) |
44.07 |
|
TSS (mg/L) |
116.58 |
|
TP (mg/L) |
11.91 |
|
TDS (mg/L) |
2842.25 |
|
Turbidity (NTU) |
271.90 |
|
Conductivity (µS/cm) |
4783.51 |
|
Colour (HU) |
104.76 |
Analytical Methods
The pH was measured with help of a digital pH meter of Elico (Model: LI614). The conductivity of the textile wastewater was determined by using a digital conductivity meter of Hanna instruments (Model: HI9932). The accuracy of pH and conductivity were continuously corrected by both sensors in correspondence with the temperature. The values of turbidity were measured using a (HACH, Model: 2100Q) turbidity meter according to the standard methods of APHA 15. The accuracies of determination of conductivity and turbidity were 0.5% to 1.0% and 0.5 NTU to 2.0 NTU, respectively. The concentration of COD was measured by using a titrimetric analysis by digestion of 20 ml of sample in addition to 10 ml of 0.025N KMnOâ‚„, a pinch of Hg2SO4 and 30 ml of H2SO4, followed by the total contents were digested for 2 hrs in a COD digester. After the digestion, the contents were diluted to 150 ml of distilled water and then it titrated against 0.1N (NH4)2Fe(SO4)2·6H2O by adding 2-3 drops of ferroin indicator. The following formula was used to determine COD values from textile wastewater.
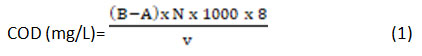
Where, B is ml of titrant used against blank. A is ml of titrant used against sample and V is the ml of sample used for titration.
BOD of the textile wastewater was measured by the titrimetric method. The dilution of 200 ml of sample in 1000 ml of distilled water were made for determining dissolved oxygen (DO) of the blank and sample and same DO of blank and samples were measured after the 5 days of incubation by adding 2 ml of each manganous sulphate and alkaline iodide azide in 300 ml of blank and sample in BOD bottle. After that, the developed precipitate was dissolved by adding 2-3 drops of sulphuric acid. Then the 50 ml of both blank and sample were titrated against 0.025N sodium thiosulphate by adding 2-3 drops of starch indicator. After DO calculation of the textile sample, the BOD was calculated from the below formula.
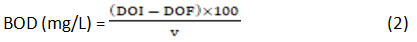
Where, DOI is DO of the sample at 0 day, DOF is DO of the sample on 5th day and V is the volume of the Sample.
Nitrate-Nitrogen was determined by using a colorimetric method by the absorbance was taken at 410 nm on UV-Visible spectrophotometer of Elico (Model SL159). Analysis of total phosphorous was done with a colorimetric method by taking absorbance at wavelength of 690 nm on a UV-Visible spectrophotometer (Elico, Model: SL159). The variations of colour in textile wastewater were measured by using a UV-Visible spectrophotometer (Elico, Model: SL159). Colour concentration was measured at a wavelength of 436 nm and values were calculated by comparing absorbance value with a standard curve. The physical parameters like. TSS, TDS, MLSS and MLVSS were analyzed by using standard methods of APHA 15.
Design of SMBR Model
The pilot-scale SMBR model having an active volume of 75 L was designed and manufactured at the School of Environmental and Earth Sciences, K.B.C.N.M. University, Jalgaon (MS) India. Figure 2 shows an experimental set-up of the SMBR model. The material utilized for the fabrication of the SMBR model was bought from the local market. Acrylic sheets were used for the manufacturing of the tank and stainless-steel valves, meters and PVC pipes were utilized for necessary requirements. The SMBR model having a total area of 19.03 m2 which containing three compartments with an area of 6.34 m2 for each compartment. The first compartment has made-up for the feed/neutralization tank in which the feed pump with a manometer and a mechanical stirrer was attached to continuous mixing and aeration of the influent. An ultrafiltration membrane made-up with polyethersulfone (PES) with an average pore size of 0.02 μm and a surface area of 0.038 m2 with inner and outer diameter of 0.6 and 0.8 mm was placed at the bottom of second compartment which has been named as ‘SMBR tank’. Also, an air sparger was placed below the membrane with an average flow rate of 3 L/min. The suction pump with the attached vacuum gauge was used to pump the influent passing through the membrane. The operational time was set at the 10 min of run and 30 seconds break for backwashing of membrane. The water level control was fixed in the SMBR tank to maintain a constant level of wastewater and HRT. The transmembrane pressure (TMP), permeate flux, physicochemical and other parameters were continuously monitored and recorded for evaluation of the overall performance of SMBR model.
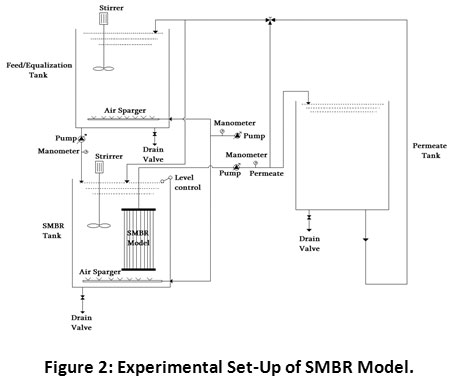 |
Figure 2: Experimental Set-Up of SMBR Model. Click here to view Figure |
Operational Condition and Monitoring of Performance
The entire operation of the SMBR model was conducted in the School of Environmental and Earth Sciences, K.B.C.N.M. University, Jalgaon (MS) India. At the beginning of operation, the activated sludge was collected from M/s. SMS WALUJ CETP Private Limited, Aurangabad, M.S., India for proper growth of bacteria in the bioreactor. The SMBR model was continuously run by feeding textile wastewater from neutralization/influent storage tank for 90 days with different HRTs of 08, 06 and 04 h, respectively. The average flux of the permeate pump was set to 19 L/m2/h and the capacity of SMBR reactor was 240-310 L/day. The COD and F/M ratio were maintained at 854-1152 mg/L and 0.08-0.17 g BOD/g MLSS/d for estimation of effluent quality at steady operation. The MLSS and MLVSS concentrations were ranges from 11,325-12,247 and 7,023-7,875 mg/L. Table 4 shows the working parameters of SMBR model. The 30 seconds of regular backwashing was applied after each 10 min of permeate flow. The SRT was countless throughout the entire operation since there was no ejection of biomass. The operational parameters of SMBR model and physicochemical characteristics of textile wastewater were continuously monitored during the overall operation period.
Table 4: Operational Conditions of SMBR Model.
|
Parameters |
HRT, h |
||
|
08.00, h |
06.00, h |
04.00, h |
|
|
Days |
1-30 |
31-60 |
61-90 |
|
TMP (psi) |
1.9-2.5 |
2.3-3.4 |
2.5-3.5 |
|
Permeate flux (L/m2 h) |
19.4-24.3 |
16.4-23.7 |
14-20.8 |
|
F/M ratio (g BOD/g MLSS. d) |
0.082-0.141 |
0.081-0.174 |
0.082-0.154 |
|
MLSS (mg/L) |
9412 |
10261 |
11039 |
|
OLR (kg BOD/m³. d) |
0.317-0.699 |
0.567-1.213 |
0.913-1.854 |
|
BOD influent (mg/L) |
406-471 |
402-452 |
401-441 |
Results and Discussion
Hollow Fibre Characterization
The cross-section and outer surface morphological properties of the polyethersulfone membrane were examined by FESEM. The interior core of the polyethersulfone membrane has distinctly observed in Figure 3a. In the cross-section of membrane, there are three layers of hollow fibre were observed. There is a finger-like structure observed near the inner and outer surface and the ellipsoid-like structure was observed to the near middle of hollow fibre cross-section. Figure 3b clearly shows the skinless porous surface on the outer surface of the polyethersulfone membrane. Hence, it has been widely chosen for wastewater treatment because of its high performance towards the removals of organics as well as suspended matter. Fig. 4 shows the EDAX spectra of the nascent polyethersulfone membrane in which the elemental composition was found to be C: 74.83%, O: 19.27% and S: 6.30%., respectively. Therefore, it has been broadly selected for the treatment of wastewater for the reason that it gives high performance regarding the removals of suspended matter as well as organics.
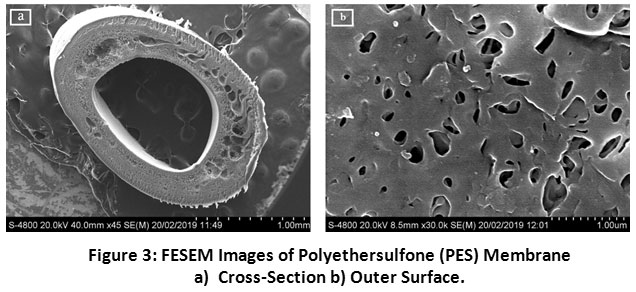 |
Figure 3: FESEM Images of Polyethersulfone (PES) Membrane (a) Cross-Section (b) Outer Surface. Click here to view Figure |
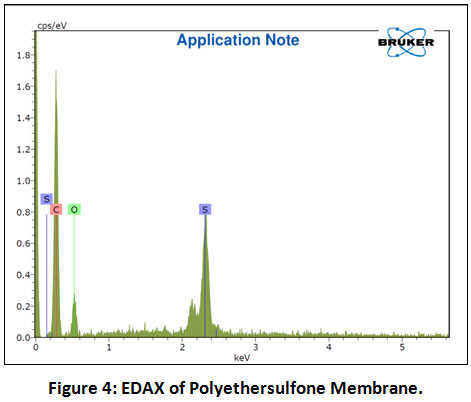 |
Figure 4: EDAX of Polyethersulfone Membrane. Click here to view Figure |
The contact angle was utilized to know the hydrophilicity/hydrophobicity of the membrane surface and it was estimated by measuring the contact angle between the membrane surfaces and water droplets. The lower contact angle indicates higher hydrophilicity and it indirectly decreases the fouling affinity 16. Figure 5 shows the measured water contact angle of the polyethersulfone hollow fibre membrane. The average membrane contact angle was found to be 62.9°. It has been clearly shown that the polyethersulfone hollow fibre membrane has a stronger hydrophilic character and it was also indicated that, it produces a fixing of particles additionally emphasized on the surface of the membrane.
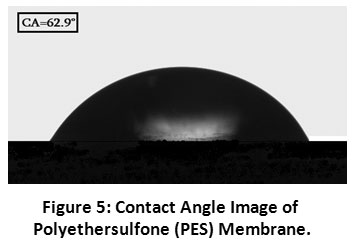 |
Figure 5: Contact Angle Image of Polyethersulfone (PES) Membrane. Click here to view Figure |
COD and BOD Removal
In the SMBR operation, HRT is the most significant variable which is associated with not only the membrane performance but also the volume and design of SMBR. Therefore, defining the ideal HRT for SMBR is very essential. As compared to the HRT of conventional biological treatment process (15-28 h), the MBR operation requires lower HRT (8-10 h) 17. As per the reliable studies reported by 18–21, an attempt was made in the present study with the HRT of 08, 06 and 04 h, respectively. The influent and effluent values of measured parameters are shown in Table 5. During the overall performance of SMBR, except TP, TDS and conductivity, the COD, BOD, NO3-N, TSS, turbidity and colour was achieved maximum treatment efficiency. The percent removal, influent and effluent values of organic pollutants i.e. COD and BOD are illustrated in Figure 6 and 7, respectively. During the SMBR performance at 08.00, 06.00 and 04.00 h HRTs, an average COD removal was achieved to be 86.69%, 84.63% and 77.51% (Table 5), respectively. At the same time, an average BOD removal was achieved to be 87.02%, 86.62% and 84.62% at 08.00, 06.00 and 04.00 h HRTs, respectively. Except for the 06.00 and 04.00 h, HRTs, the highest removal of COD and BOD was achieved. Similarly, Friha et al also reported that the pilot-scale membrane bioreactor (MBR) system has achieved the highest removal of COD and BOD was about 98% and 96%, respectively 22. It has been indicated that the SMBR has approximately removed all the biodegradable pollutants.
Table 5: Influent and Effluent Values of Textile Wastewater during the Performance of SMBR Model.
|
Parameters |
Influent, average (min.-max.) |
Effluent, average (min.-max.) |
|
COD (mg/L) |
997 (854-1152) |
168 (110-241) |
|
BOD (mg/L) |
421 (401-471) |
58 (45-76) |
|
NO3-N (mg/L) |
44 (36-51) |
9 (6-13) |
|
TSS (mg/L) |
116 (102-134) |
2 (0.5-3.7) |
|
TP (mg/L) |
11 (8-17) |
4 (3-7) |
|
TDS (mg/L) |
2842 (954-4951) |
2784 (912-4883) |
|
Turbidity (NTU) |
271 (232-316) |
7 (3-10) |
|
Conductivity (µS/cm) |
4783 (1461-8628) |
4657 (1382-8437) |
|
Colour (HU) |
104 (86-129) |
22 (10-38) |
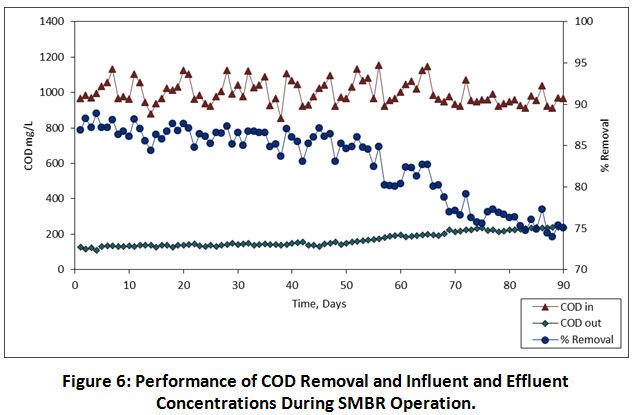 |
Figure 6: Performance of COD Removal and Influent and Effluent Concentrations During SMBR Operation. Click here to view Figure |
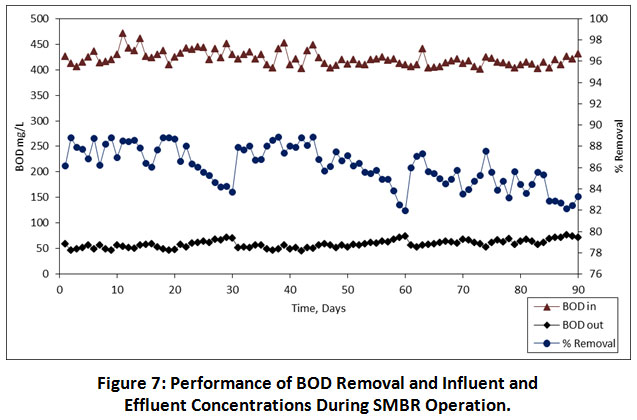 |
Figure 7: Performance of BOD Removal and Influent and Effluent Concentrations During SMBR Operation. Click here to view Figure |
Nitrate-Nitrogen Removal
The influent concentration of NO3-N was ranging from 36-51 mg/L (Table 5). During the entire operation of SMBR, a biofilm was gradually developed on the membrane and the concentration of NO3-N in the effluent was gradually dropped to 6-13 mg/L, respectively. As shown in Figure 8, an average removal of NO3-N during the entire operation at 08.00, 06.00, 04.00 h, HRTs were 82.44%, 79.39% and 75.63%, respectively. Similarly, Xia et al also reported that they achieved an average NO3-N removal was about 95.1% while treating the contaminated drinking water by using HBPC-MBR.23
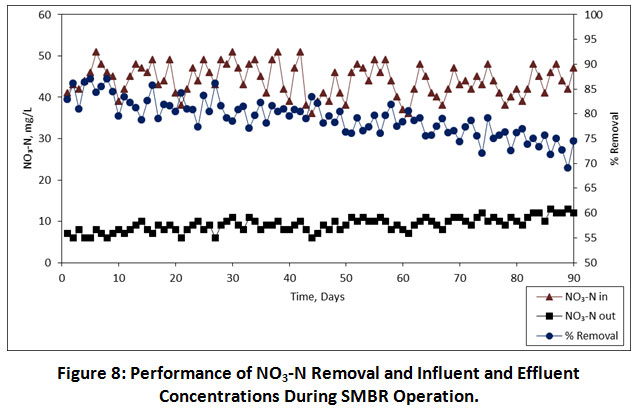 |
Figure 8: Performance of NO3-N Removal and Influent and Effluent Concentrations During SMBR Operation. Click here to view Figure |
Turbidity and TSS Removal
The performance of TSS removal in the SMBR reactor was found to be in a very high amount i.e. 98.79%, 98.24% and 97.57% at 08.00, 06.00, 04.00 h, HRTs, respectively. An average influent concentration of TSS were ranges from 102-134 mg/L, while the effluent concentration of TSS was gradually decreased in the range of 0.5-3.7 mg/L. The variations of TSS removal and concentrations of influent and effluent during the entire operation are illustrated in Figure 9. Similarly, the turbidity was also removed in a high amount. The turbidity concentrations in the influent were ranges from 232-316 NTU. The average turbidity removal at 08.00, 06.00, 04.00 h, HRTs was found to be 98.16%, 97.40% and 96.91%, respectively. Figure 10 shows variations of turbidity removal and concentrations of influent and effluent during the entire operation. During the entire operation, the performance of both TSS and turbidity removal was not affected by the operational conditions. These results indicated that the SMBR has major advantages to the removal of high amounts of organics and particulate matter.
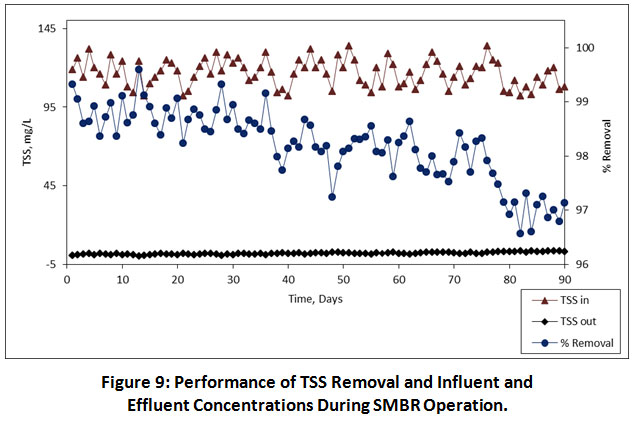 |
Figure 9: Performance of TSS Removal and Influent and Effluent Concentrations During SMBR Operation. |
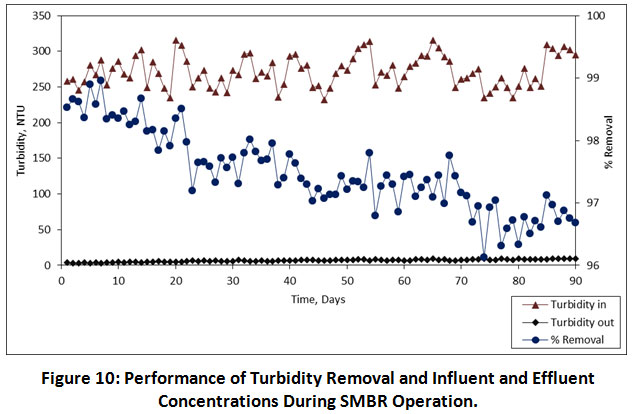 |
Figure 10: Performance of Turbidity Removal and Influent and Effluent Concentrations During SMBR Operation. Click here to view Figure |
Colour Removal
The colour is a physicochemical parameter that can be easily determined by analytical methods and it gives valuable detail about the quality of water. Figure 11 shows variations of dye removal and concentrations of dyes in influent and effluent. The performance of percentage colour removal at 08.00, 06.00, 04.00 h, HRTs was found to be 83.52%, 81.23% and 72.33%, respectively. It has been shown that the SMBR retains active microorganisms that produce extracellular enzymes which degrade organics in the wastewater. Similarly, Aouni et al were reported that the nanofiltration membrane can remove >90% of colour in the textile wastewater 24. Also, Zheng et al have reported a study on the removal of colour with submerged nanofiltration membrane bioreactor, which has 99.3% of colour removal was achieved 25. The HRT of 08.00 h has achieved maximum colour removal, but only 2% greater than the 06.00 h HRT. From a technical perspective, the lower HRT is suggested due to its minimizes not only the biomass but also the size of bioreactor, which will be important to the degradation of organics. As per the results obtained from the present study, it has been indicated that the HRT of 06.00 h is an optimum HRT for the removal colour in the textile wastewater.
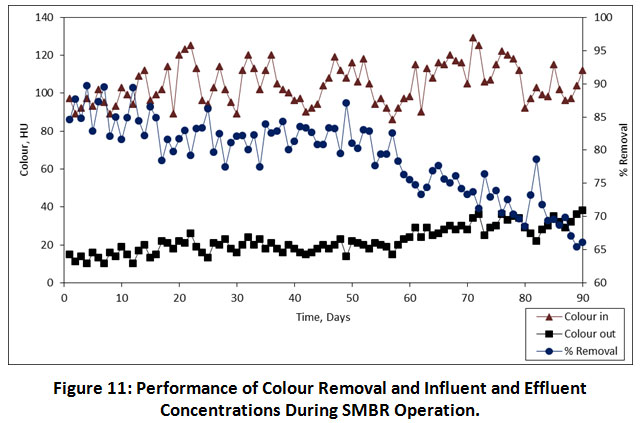 |
Figure 11: Performance of Colour Removal and Influent and Effluent Concentrations During SMBR Operation. Click here to view Figure |
Effects of OLR on COD Removal
The effects of OLR on COD removal has illustrated in Figure 12. It was indicated that the overall performance of COD removal was not adversely affected by the OLR variations. Similarly, Baêta et al also reported that the OLR in the MBR reactor has does not affect to removal efficiency of COD26. During the entire operation of SMBR at 08.00, 06.00 and 04.00 h HRTs, the OLR was in the range of 0.317-0.699, 0.567-1.213 and 0.913-1.854 kg BOD/m3. d.
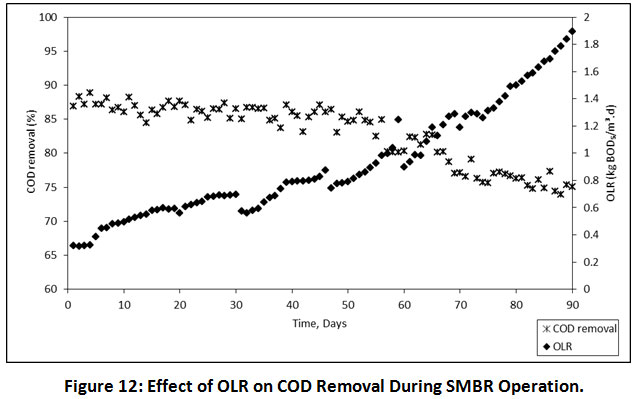 |
Figure 12: Effect of OLR on COD Removal During SMBR Operation. Click here to view Figure |
Effects of F/M ratio on BOD Removal
The F/M ratio in the conventional activated sludge system fell in the range of 0.07 to 1.6/d, but in the SMBR operation, it is typically found to be <0.1/d18. The microbial activity in the SMBR remains high because of their rejection by the membrane. The highest BOD removal and decent settleability of sludge were obtained at a low food/biomass ratio. In our study, the F/M ratio fell in the range of 0.082-0.141, 0.081-0.174 and 0.082-0.154 g BOD/g MLSS. d at 08.00, 06.00 and 04.00 h HRTs, respectively. The effects of F/M ratio on BOD has illustrated in Figure 13. The high amount of MLSS in the SMBR decreases the F/M ratio. Hence, At a low F/M ratio and high micro-organisms per unit volume achieves a higher reduction of BOD. The F/M ratio also restraints biomass properties which are indirectly affected the fouling susceptibility of a membrane27.
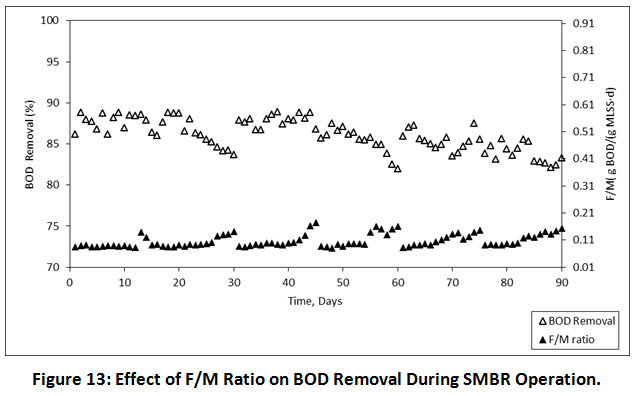 |
Figure 13: Effect of F/M Ratio on BOD Removal During SMBR Operation. Click here to view Figure |
Permeate Flux, Transmembrane Pressure and Membrane Cleaning
The variations of TMP during 08.00, 06.00, 04.00 h, HRTs were found in the range of 1.9-2.5, 2.3-3.4 and 2.5-3.5 psi, respectively. It was occurred due to the membrane was moderately fouled which results in a gradual decrease of permeate flux. This has repeatedly occurred during the overall performance. For that, 30 seconds of routine backwashing after each 10 min of the run was applied to the membrane. Also, at an interval of every fifteen days, an ex-situ chemical cleaning was done by soaking the membrane with citric acid and sodium hypochlorite for about 5 h. After cleaning of membrane, the TMP was gradually decreased from 3.5 to 2.3 psi and permeate flux was reached up to 23 L/m2/h. The average permeate flux at 08.00, 06.00, 04.00 h, HRTs was found in the range of 19.4-24.3, 16.4-23.7 and 14-20.8 L/m2/h, respectively. Figure 14 illustrates the variations of TMP and permeate flux during the overall operation period. It has been indicated that the regular chemical cleaning increases the permeability of membrane and it retains constant permeate flux.
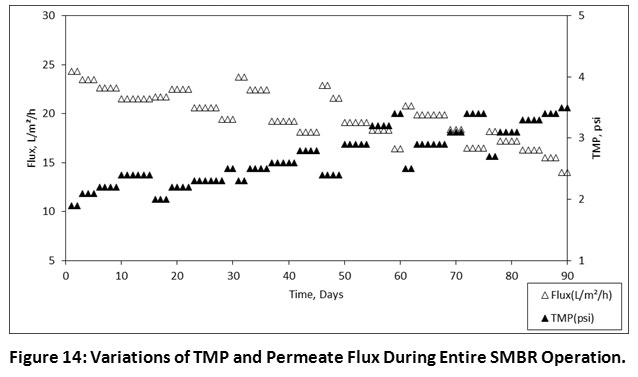 |
Figure 14: Variations of TMP and Permeate Flux During Entire SMBR Operation. Click here to view Figure |
Conclusions
Throughout the entire operation of the SMBR model for treating the textile wastewater, except the TP, TDS and conductivity, an > 84% of removal for COD, BOD, NO3-N, TSS, turbidity and colour was achieved. The SMBR performance was not severely affected by the increasing amount of OLR, F/M ratio and MLSS concentrations. However, except for the 04.00 h HRT, the removal efficiency of COD, BOD, NO3-N, TSS, turbidity and colour was obtained at 06.00 h and 08.00 h, HRTs were more than 84%, respectively. It has been concluded that the outcome of the present study indicates the 06.00 h, HRT would be the best optimum conditions for the treatment of textile wastewater. The combination of a high amount of MLSS and microorganisms was significantly achieved denitrification and removes an average of 86% NO3-N and 89% colour. The utilization of chemical cleaning and application of continuous backwashing during the entire SMBR operation has significantly prevented the membrane from severe fouling which resulting an average permeate flux of 19 L/m2/h. The membrane was moderately fouled during the 86-90 days of operation and TMP was reached up to 3.5 psi. it was also concluded that the 89% colour removed wastewater would be again reusable for different processes in the textile industry. The unremovable parameters like. TP, TDS and conductivity need further treatment like. Reverse osmosis or nanofiltration. The outcomes from the present study revealed that the pilot-scale SMBR can effectively treat the textile wastewater and it has been recommended for the large-scale operation of textile wastewater treatment.
Acknowledgment
The author (Ganpat More) are thankful to Shri. G.H. Raisoni Foundation for giving financial support to this research work. Author also thankful to the Director, School of Environmental and Earth Sciences, Kavayitri Bahinabai Chaudhari North Maharashtra University, Jalgaon for providing necessary facilities.
Nomenclature
- SMBR: submerged membrane bioreactor
- HRT: hydraulic retention time
- TDS: total dissolved solids
- TSS: total suspended solids
- MLSS: mixed liquor suspended solids
- MLVSS: mixed liquor volatile suspended solids
- COD: chemical oxygen demand
- BOD: biochemical oxygen demand
- TP: total phosphorus
- OLR: organic loading rate
- PES: polyethersulfone
- TMP: transmembrane pressure
- F/M ratio: food/microorganisms ratio
References
- Sahinkaya E, Uzal N, Yetis U, Dilek FB. Biological treatment and nanofiltration of denim textile wastewater for reuse. J Hazard Mater. 2008;153(3):1142-1148. doi:10.1016/j.jhazmat.2007.09.072.
CrossRef - Smith B. Pollution Prevention Needs and Opportunities in the Textile Industry.; 1994.
- Bertea AF, Butnaru R. Polyamide dyeing wastewater recycling after Fenton-like oxidative treatment. Ind Textila. 2012;63(6):322-326.
- Shahf SFA, Shahf AK, Mehdi A, Memon AA, Harijan K, Ali ZM. Analysis and treatment of industrial wastewater through chemical coagulation-adsorption process- A Case Study of Clariant Pakistan Limited. AIP Conf Proc. 2011;1453(1):353-358. doi:10.1063/1.4711199.
CrossRef - Verma AK, Dash RR, Bhunia P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manage. 2012;93(1):154-168. doi:10.1016/j.jenvman.2011.09.012.
CrossRef - Spagni A, Grilli S, Casu S, Mattioli D. Treatment of a simulated textile wastewater containing the azo-dye reactive orange 16 in an anaerobic-biofilm anoxic-aerobic membrane bioreactor. Int Biodeterior Biodegrad. 2010;64(7):676-681. doi:10.1016/j.ibiod.2010.08.004.
CrossRef - Pang YL, Abdullah AZ. Current status of textile industry wastewater management and research progress in malaysia: A review. Clean - Soil, Air, Water. 2013;41(8):751-764. doi:10.1002/clen.201000318.
CrossRef - Aplin R, Waite TD. Comparison of three advanced oxidation processes for degradation of textile dyes. Water Sci Technol. 2000;42(5-6).
CrossRef - Demirbas A. Agricultural based activated carbons for the removal of dyes from aqueous solutions: A review. J Hazard Mater. 2009;167(1-3):1-9. doi:10.1016/j.jhazmat.2008.12.114.
CrossRef - Yurtsever A, Sahinkaya E, AktaÅŸ Ö, Uçar D, Çinar Ö, Wang Z. Performances of anaerobic and aerobic membrane bioreactors for the treatment of synthetic textile wastewater. Bioresour Technol. 2015;192:564-573. doi:10.1016/j.biortech.2015.06.024.
CrossRef - Judd S, Judd C. The MBR Book: Principles and Applications of Membrane Bioreactors in Water and Wastewater Treatment.; 2008. doi:10.1016/B978-185617481-7/50005-2.
CrossRef - Schoeberl P, Brik M, Braun R, Fuchs W. Treatment and recycling of textile wastewater - Case study and development of a recycling concept. Desalination. 2005;171(2):173-183. doi:10.1016/j.desal.2004.02.105.
CrossRef - Yu S, Liu M, Ma M, Qi M, Lü Z, Gao C. Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration membranes. J Memb Sci. 2010;350(1-2):83-91. doi:10.1016/j.memsci.2009.12.014.
CrossRef - Kim JH, Lee KH. Effect of PEG additive on membrane formation by phase inversion. J Memb Sci. 1998;138(2):153-163. doi:10.1016/S0376-7388(97)00224-X.
CrossRef - APHA. Standard Methods for the Examination of Water and Wastewater. 21th ed Am Public Heal Assoc Washingt DC, USA. 2012. doi:10.1080/19447013008687143.
CrossRef - Norberg D, Hong S, Taylor J, Zhao Y. Surface characterization and performance evaluation of commercial fouling resistant low-pressure RO membranes. Desalination. 2007;202(1-3):45-52. doi:10.1016/j.desal.2005.12.037.
CrossRef - Yigit NO, Uzal N, Koseoglu H, et al. Treatment of a denim producing textile industry wastewater using pilot-scale membrane bioreactor. Desalination. 2009;240(1-3):143-150. doi:10.1016/j.desal.2007.11.071.
CrossRef - Badani Z, Ait-Amar H, Si-Salah A, Brik M, Fuchs W. Treatment of textile waste water by membrane bioreactor and reuse. Desalination. 2005;185(1-3):411-417. doi:10.1016/j.desal.2005.03.088.
CrossRef - Tay JH, Luhai Zeng J, Sun DD. Effects of hydraulic retention time on system performance of a submerged membrane bioreactor. In: Separation Science and Technology. Vol 38. ; 2003:851-868. doi:10.1081/SS-120017630.
CrossRef - Ünlü A, Hasar H, Kinaci C, Çakmakci M, Koçer NN. Real role of an ultrafiltration hollow-fibre membrane module in a submerged membrane bioreactor. Desalination. 2005;181(1-3):185-191. doi:10.1016/j.desal.2005.02.020.
CrossRef - Schoeberl P, Brik M, Bertoni M, Braun R, Fuchs W. Optimization of operational parameters for a submerged membrane bioreactor treating dyehouse wastewater. Sep Purif Technol. 2005;44(1):61-68. doi:10.1016/j.seppur.2004.12.004.
CrossRef - Friha I, Bradai M, Johnson D, et al. Treatment of textile wastewater by submerged membrane bioreactor: In vitro bioassays for the assessment of stress response elicited by raw and reclaimed wastewater. J Environ Manage. 2015;160:184-192. doi:10.1016/j.jenvman.2015.06.008.
CrossRef - Xia S, Zhang YH, Zhong FH. A continuous stirred hydrogen-based polyvinyl chloride membrane biofilm reactor for the treatment of nitrate contaminated drinking water. Bioresour Technol. 2009;100(24):6223-6228. doi:10.1016/j.biortech.2009.07.002.
CrossRef - Aouni A, Fersi C, Cuartas-Uribe B, Bes-Pía A, Alcaina-Miranda MI, Dhahbi M. Reactive dyes rejection and textile effluent treatment study using ultrafiltration and nanofiltration processes. Desalination. 2012;297(June):87-96. doi:10.1016/j.desal.2012.04.022.
CrossRef - Zheng Y, Yu S, Shuai S, et al. Color removal and COD reduction of biologically treated textile effluent through submerged filtration using hollow fiber nanofiltration membrane. Desalination. 2013;314:89-95. doi:10.1016/j.desal.2013.01.004.
CrossRef - Baêta BEL, Lima DRS, Queiroz Silva S, Aquino SF. Influence of the applied organic load (OLR) on textile wastewater treatment using submerged anaerobic membrane bioreactors (SAMBR) in the presence of redox mediator and powdered activated carbon (PAC). Brazilian J Chem Eng. 2016;33(4):817-825. doi:10.1590/0104-6632.20160334s20150031.
CrossRef - Zhang J, Chua HC, Zhou J, Fane AG. Effect of sludge retention time on membrane bio-fouling intensity in a submerged membrane bioreactor. Sep Sci Technol. 2006;41(7):1313-1329. doi:10.1080/01496390600683647.
CrossRef







