Tolerance of Three Ornamental Plant Species to Chromium contamination in Soil and their Potential for Phytoextraction and Phytostabilization of the Toxic Metal
DOI: http://dx.doi.org/10.12944/CWE.16.2.06
Copy the following to cite this article:
Sehrawat G, Singh R, Kaushik A. Tolerance of Three Ornamental Plant Species to Chromium contamination in Soil and their Potential for Phytoextraction and Phytostabilization of the Toxic Metal. Curr World Environ 2021;16(2). DOI:http://dx.doi.org/10.12944/CWE.16.2.06
Copy the following to cite this URL:
Sehrawat G, Singh R, Kaushik A. Tolerance of Three Ornamental Plant Species to Chromium contamination in Soil and their Potential for Phytoextraction and Phytostabilization of the Toxic Metal. Curr World Environ 2021;16(2). Available From : https://bit.ly/3yeYUFu
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2021-01-23 |
|---|---|
| Accepted: | 2021-07-03 |
| Reviewed by: | 
 Yulianto Suteja
Yulianto Suteja
|
| Second Review by: |

 Prabhu K
Prabhu K
|
| Final Approval by: | Dr. Hemant Kumar |
Introduction
Various anthropogenic activities as well as geological processes lead to chromium contamination of soils and being non-biodegradable, the metal persists in the soil system for years, affecting soil quality and plant life. Use of chromium in several industries like electroplating, manufacturing alloy products, nuclear reactor vessels, leather tanning,textile and dye synthesis ultimately leads to its discharge in the wastewaters and sludge that impacts both aquatic and terrestrial ecosystems 1. Out of the two forms of chromium, Cr (III) occurs naturally in soil and is used by organisms as a micro-nutrient for their growth and development 2,whereas Cr(VI) is a potent toxin that is produced by various anthropogenic activities and also, by natural oxidation of Cr(III). Hexavalent chromium is extremely reactive and hazardous in nature. It has been found that 75-100 ppm concentrations generally do not adverselyaffect plant growth, but above this concentration it is toxic and inhibitory 3. Concentration of Cr in contaminated soils, particularly in industrial and mined areas often exceeds 1000ppm. Therefore, it becomes particularly important to decontaminate soils that have concentrations of Cr exceeding 100ppm. Various conventional methods used for removal of contaminants at large scale are not only expensive but also affect soil constitution and its fertility 4. While using a method for soil decontamination, it is important to see that it does not lead to pollution of other environmental components such as air or water. Phytoremediation has emerged as one such method that is less expensive as well as eco-friendlyinnature.Phytoextraction and phytostabilization are the twoimportantstrategies that are useful for the phytoremediation of metal contaminated soils.
Phytoextraction has been widely studied because metal removal from polluted soils in such systems is high and economic. However, when the metals present in the soil get accumulated in plants, there are chances of their getting transferred through the food chain. It is, therefore proposed to use such plants for phytoremediation, which do not contribute directly towards food production and hence can be used in an environmentally safe manner. A few studies using ornamentalssuch as Tagetes erecta and Helianthus annus thathave shown promiseor phytoremediation of heavy metals like zinc and cadmium 5,6. Considering the enormous diversity of plants and their wide range of metal toleranceand accumulation capacity,it is worthwhile to explore newer species that might prove useful in phytoremediation.
The present study is aimed at exploring Cr toleranceand phytoremediation potential ofsomefast-growingherbaceous ornamental plants (Sansevieria trifasciata var. hahnii,Canna indica (L.) and Nephrolepis exaltata (L.), whichpossess good biomass with a robust root system.Based on a preliminary screening study using a variety of plant species for extraction ofmulti metals, these plants were selected.7 These plants showed metal uptake capacity and were easy to grow, and their perennial nature provides environmentally sound long-term phytoremediation. Besides, they add to the aesthetics and also have very little chances of transfer of the metals through food chain.
Considering the fact that tolerance of the species to high concentrations of the metal and good biomass would be vital in this context, plant growth, aboveground and belowground biomass, biochemical parameters like chlorophyll, carotenoids and oxidative enzymes like superoxide dismutase(SOD) and catalase(CAT) were studied in response to a range of Cr (VI) concentrations and bioremediation potential was assessed by determining their bioaccumulation and bio-concentration factors.
Methodology
Pot-Culture Experiments
The plant species selected for the study, namely, Sansevieria trifasciata var. hahnii, commonly known as bird’s nest snake plant (family Asparagaceae), Canna indica (L.)commonly known as Indian shot (Cannaceae) and Nephrolepis exaltata (L.) known as Boston fern or sword fern (Nephrolepidaceae) are grown in the tropics and sub-tropics as ornamental species. These plants have good biomass and are not used as a food resource in these regions.
Plants of all the three species were obtained from a local nursery in New Delhi, India and young plants approximately of same age, height and biomass were selected for the experiments. Pot culture experiments were carried out in greenhouse within the university campus under controlled conditions (270C ± 30C). Different concentrations of Cr (250, 500, 750 mg Cr-1 kg soil) were artificially created by spiking normal garden soil with calculated amounts of K2Cr2O7, the pre-treatment soil without any Cr addition with pH 6.8 and organic carbon 0.28% served as control. A total of 72 pots (3 species x 4 treatments x 2 sampling days x 3 replicates)were taken. Each pot was filled with 5kg soil of the desired Cr concentration. The desired concentrations of Cr were added after accounting for Cr already present in the original pre-treated soil(11.6 mg Cr-1 kg soil) as determined byusing atomic absorption spectrophotometer explained ahead. Each pot was watered equally as and when required using tap water.Calculated quantity of Cr was added to fixed amount of soil in pots and saucers were placed under each pot to collect the drained water, which was then poured back to pots so that any loss of Cr leached out of the pot was restored.
Growth and Tolerance Studies
Tolerance of the plant species to different concentrations of Cr was studied in terms of their aboveground and belowground biomass, leaf chlorophyll, carotenoid content and anti-oxidative enzyme activities. Plants were harvested twice (30d, 60d after transplantation),washed in deionized water and separated into aboveground and belowground parts. Harvesting of the plants was done on 30 and 60 days after sowing, when the plants showed good foliage growth. In a preliminary screening experiment these plant species were selected and their harvesting time were decided on the basis of the results obtained on metal uptake.Plant parts were separated into aboveground and belowground parts,the separated parts were dried in oven at 75°C for 48h. The samples were then weighed on electronic balance to obtain dry weight of plant parts independently and was expressed as g per plant.
Estimation of total chlorophyll contents in leaves was done following protocol given by Arnon 8and carotenoids by following Lichtenthaler9.0.5 gram of fresh leaves samples were cut into small pieces and macerated with 80% acetone for a few minutes. The homogenate was then centrifuged at 10,000g. Supernatant was then separated and analysed for the chlorophyll concentration by reading the absorbance of the supernatant on UV-vis spectrophotometer at 470, 645 and 663nm, using the formulae:
Chl a (mgg-1fw) = [12.7 (A663) -2.69 (A645)] x V/ 1000 x W
Chl b (mgg-1fw) = [22.9 (A645)- 4.68 (A663)] x V/ 1000 x W
Total Chlorophyll (mgg-1fw) = [20.2 (A645)– 8.02 (A663)] x V/ 1000 x W
Carotenoids (mgg-1fw) = [1000 A470 -1.82 x Chl a -85.02 Chl b] x V/ 198x1000xW
V-volume of the sample (ml)
W-Fresh weight of the sample (g)
Antioxidative enzymes, Superoxide dismutase (SOD) and catalase (CAT) for both aboveground and belowground parts were assayed considering their role in metal tolerance. Superoxide dismutase activity was determined at 4°C following Nishikimi 10as modified by Kakkar 11and SOD activity is expressed as U g-1 FW where one unit of SOD activity refers to the amount of enzyme required for 50 % inhibition of NBT reduction under assay conditions.For the assessement of superoxide dismutase (SOD) enzyme activity, samples were macerated in 0.1 M phosphate buffer (pH 7.5) and centrifuged at 10,000 g for 20 min at 4°C. Supernatant was used then analysed for enzyme activity.The assay mixture for SOD activity contained Tris-HCl buffer, Phenazine methosulfate (PMS), NBT and NADH and cell free extract. The reaction was terminated by adding galcial acetic acid and absorbance was taken at 560nm.
Catalase activity was determined following the method of Sinha12. The assay mixture was composed of 0.1 M Phosphate buffer, potassium dichromate:glacial acetic acid (1:3), 0.2 M H2O2 and enzyme extract.The absorbance was taken at 570 nm. One unit of enzyme activity was defined as the amount of enzyme which catalyzed the oxidation of 1 mmole H2O2 per minute under assay condition.
Metal Analysis and Accumulation Factors in Plants
Dried samples of plants (aboveground and belowground parts) were ground into fine powder and 0.5 g of each sample was digested using HNO3 and HClO4 (5:1 v/v) by heating on an electric hot plate at 80-90°C till a clean solution was obtained. The samples were cooled and filtered through Whatman 42 filter paper, and diluted to 50 ml using deionised water and analyzed for total Cr using atomic absorption spectroscopy (AAS-Agilent 280 FS AA)13. Different concentrations of Cr were prepared from the standard solution (1000 mg/l ; Sigma-Aldrich) using deionized water for dilution and a calibration curve was drawn using the known concentrations and absorbance data. Cr Concentration in the samples were read from the calibration curve using its absorbance. For background correction a blank without the analyte was run in each analytical batch.
Bioaccumulation factor (BAF) was calculated as: Crconcentration in plant shoot/ Cr concentration insoil14. Bioconcentration factor (BCF) was calculated as ratio of the metal concentration in plant roots to that in soil 15. Phytoextraction capacity of the plants was the total metal extracted from the soil by the plant, and was calculated as the product of biomass of plant parts and metal concentration in plant parts.
The total metal concentration in soil was determined following Allen16 by digesting 0.5g of dried and sieved soil samples with concentrated HNO3, H2SO4 and HClO4 (5:1:1) at 80°C and digested samples were then filtered through Whatman 42 filter paper and filtrates were diluted to 50 ml with deionized water.
Statistical Analysis
The data were analyzed to test the statistical significance of differences in various parameters in response to different Cr concentrations using one way ANOVA, followed by Tukey’s test for comparison of individual means using SPSS software (20.0 version).
Results
Plant Response to Varying Cr Concentrations in Soil
All the plant species grew very well in the greenhouse in Cr contaminated soils in pots. S. trifasciata was found to have maximum aboveground biomass (25g) while C.indica had maximum belowground biomass (25g) under control conditions (Table 1). In comparison to control, Cr250 did not show any significant adverse effect on aboveground biomass of Canna and Nephrolepis, while in case of Sansevieria,Cr250 as well as Cr500 had no adverse effect till 30d. However, a significant (p< 0.05) decline in aboveground biomass was seen at later stage (60d) in response to all Cr concentrations for all the species. The decline was more in C. indica, (32-86%), followed by N. exaltata(25-66%) and S.trifasciata (16-52%). Thus, tolerance to high Cr contamination in soil in the order Sansevieria>Nephrolepis>Cannain terms of aboveground biomass.
Table 1: Biomass of the Plant Species Exposed to Different Chromium Concentrations in Soil.
|
|
Dry biomass(g plant -1) |
||||||
|
Plant species |
Dose |
Aboveground |
Belowground |
||||
|
|
30 d |
60 d |
30d |
60 d |
|||
|
C.indica |
Control |
10.05 ± 0.64 |
14.38 ± 0.50 |
15.14± 0.93 |
17.12 ± 0.62 |
||
|
Cr250 |
9.08 ± 0.83 |
8.65 ± 0.64*** |
12.70 ± 0.59** |
11.79± .67*** |
|||
|
Cr500 |
6.91 ± 0.70** |
6.23 ± 0.94*** |
10.55 ± 0.31*** |
8.21 ± 0.22*** |
|||
|
Cr750 |
4.41 ± 0.36*** |
2.6 ± 0.42*** |
7.9 ± 0.67*** |
4.24 ± 0.19*** |
|||
|
N.exaltata |
Control |
14.62 ± 0.98 |
18.43 ± 0.31 |
12.86 ± 0.68 |
14.85 ± 0.73 |
||
|
Cr250 |
15.65 ± 0.55** |
13.76 ± 0.41*** |
11.12 ± 0.83* |
8.78 ± 0.75*** |
|||
|
Cr500 |
12.22 ± 0.41*** |
10.21 ± 0.21*** |
9.36 ± 0.32*** |
7.44 ± 0.49*** |
|||
|
Cr750 |
8.49 ± 0.43* |
6.23 ± 0.31*** |
8.79 ± 0.37*** |
6.59 ± 0.35*** |
|||
|
S.trifasciata |
Control |
25.65 ± 1.16 |
27.28 ± 1.15 |
9.54 ± 0.45 |
11.97 ± 0.75 |
||
|
Cr250 |
26.35 ± 1.62 |
22.92 ± 1.03*** |
8.26 ± 0.09* |
9.20± 0.56*** |
|||
|
Cr500 |
23.82 ± 1.67 |
19.05 ± 0.64*** |
7.41 ± 0.49*** |
6.68 ± 0.29*** |
|||
|
Cr750 |
17.17 ± 0.19*** |
13.17 ± 0.19*** |
5.68 ± 0.47*** |
3.8 ± 0.92*** |
|||
|
|
|
|
|
|
|
||
Values are mean ± standard deviation (n=3), significant difference w.r.t. control is represented as * p <0.05, **p<0.01, ***p<0.001
Belowground biomass of all the species, however, decreased on exposure to Cr from the beginning that became more pronounced at later stage. There was a decline of 51- 83% for C.indica, 31- 55% for N.exaltata and 52- 80% for S. trifasciata at different concentrationson 60d, Thus, Nephrolepis showed better tolerance to the metal than the other two ornamental species in terms of belowground biomass.
Leaf Chlorophyll and Carotenoid
Leaf chlorophyll was not affected by Cr concentrations up to 250 mgkg -1, but concentrations higher than this (Cr500,Cr750) had a significant (p<0.05) effect on leaf chlorophyll and carotenoid concentrations (Table 2) as compared to respective control in case of Canna and Nephrolepis. On the other hand, leaf chlorophyll in Sansevieria was not affected significantly even by high Cr concentrations in soil.
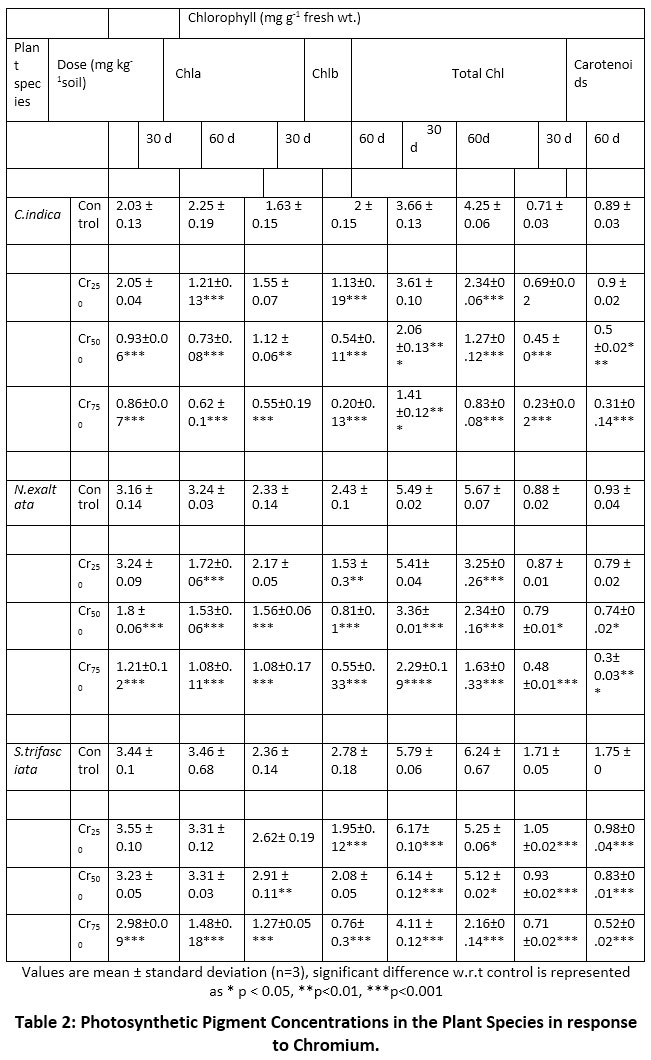 |
Table 2: Photosynthetic Pigment Concentrations in the Plant Species in response to Chromium. Click here to view Table |
Total chlorophyll concentrations (mgg-1fw) on 30 and 60d, were more in S. trifasciata, (5.8- 6.2), followed by N. exaltata (5.5 and 5.7)and were lowest in C. indica (3.7-4.3) under control conditions. On exposure to Cr (250 to 750mg kg -1), total chlorophyll (mgg-1fw) was 3.61 to 0.83(C. indica), 5.41 to 1.63 (N.exaltata) and 6.17 to 2.16(S.trifasciata), showing a major decline at Cr750.
While Chla was affected in these species, when exposed to a concentration exceeding Cr250, a significant decline was observed in Chl b even at Cr250. While the decline was 46% in C. indica and N. exaltata at Cr250, and 67-72% at Cr750, there was an increase in chlorophyll concentration in S.trifasciata on exposure up to 500 mg kg -1 in initial days, particularly. Chl b response to Cr also followed the same trend (Table 2).
Decrease in leaf carotenoid content was also observed in the presence of high concentrations of chromium. Decline in carotenoid recorded for C. indica, N.exaltata and S.trifasciata was 58, 62 and 70%, respectively at Cr 750 on 60d exposure, while at lower Cr concentrations the pigment was not adversely affected.
Antioxidative Enzyme Activities
Activities of certain anti-oxidative enzymes are required by plants to overcome the oxidative stress. Table 3 shows how the concentration of chromium and duration of exposure influenced catalase activity of the three plant species. Catalase activity was significantly higher (p<0.05) on 30d as compared to that on 60d. Catalase activity in above ground parts of C. indica was marginally less (5%) than that in control, while at Cr250, in N.exaltata and S.trifasciata, it was 16-17% less than that at control on 30d. However, at Cr750, the decline became more prominent (34- 46%),in aboveground parts for C. indica, N.exaltata and S.trifasciata on 60d. Same trend of decline was observed for belowground parts of the plants with (8-21%) at Cr250 on 30d and much greater (47-68%) at Cr750.
Table 3: Catalase Activity in Test Plant Species.
|
Catalase Activity (µ moles of H2O2 consumed min-1 g-1 FW) |
||||||||||||||
|
Plant species |
Dose (mg kg-1soil) |
Aboveground |
Belowground |
|||||||||||
|
|
30 d |
60d |
30 d |
60 d |
||||||||||
|
|
|
|||||||||||||
|
C. indica |
Control |
1.41 ± 0.01 |
1.43 ± 0.06 |
1.42 ± 0.01 |
1.45 ± 0.01 |
|||||||||
|
Cr250 |
1.35 ± 0.01 |
0.91 ± 0.02*** |
1.31 ± 0.01 |
0.80 ± 0.01*** |
||||||||||
|
Cr500 |
1.31 ± 0.01*** |
0.87 ± 0.02*** |
1.22 ± 0.01*** |
0.71 ± 0.01*** |
||||||||||
|
Cr750 |
1.27 ± 0.01*** |
0.78 ± 0.00*** |
1.09 ± 0.01*** |
0.66 ± 0.02*** |
||||||||||
|
|
|
|
|
|
|
|
||||||||
|
N. exaltata |
Control |
1.15 ± 0.02 |
1.16 ± 0.01 |
1.48±0.01 |
1.50 ± 0.02 |
|||||||||
|
Cr250 |
0.96 ± 0.01*** |
0.90 ± 0.01*** |
1.2 ± 0.02 |
0.70 ± 0.01*** |
||||||||||
|
Cr500 |
0.99 ± 0.01*** |
0.86 ± 0.02*** |
1.29 ± 0.02*** |
0.62 ± 0.01*** |
||||||||||
|
Cr750 |
0.86 ± 0.02*** |
0.77 ± 0.01*** |
1.39 ± 0.05** |
0.49 ± 0.01*** |
||||||||||
|
|
|
|
|
|
|
|
||||||||
|
S.trifasciata |
Control |
1.44 ± 0.02 |
1.46± 0.01 |
1.32 ± 0.02 |
1.33 ± 0.01 |
|||||||||
|
Cr250 |
1.19 ± 0.01 |
1.04 ± 0.01*** |
1.05± 0.01*** |
1.00 ± 0.00*** |
||||||||||
|
Cr500 |
1.25 ± 0.02*** |
0.90 ± 0.01*** |
1.08 ± 0.01*** |
0.92 ± 0.007*** |
||||||||||
|
Cr750 |
1.14 ± 0.01*** |
0.79 ± 0.01*** |
0.96± 0.01*** |
0.70 ± 0.01*** |
||||||||||
|
|
|
|
|
|
|
|||||||||
Values are mean ± standard deviation (n=3), significant difference w.r.t. control is represented as * p < 0.05, **p<0.01, ***p<0.001
Another antioxidant enzyme Superoxide dismutase on exposure to Cr250,however showed increased activity in all the three species (Table 4). At Cr250,SOD activity was boosted by 84% in in aboveground parts of Canna, and by7-35% in the other two species, whereas it was increased by 86% in belowground parts of Nephrolepis, and 12-23% in the others.
Table 4: Superoxide Dismutaseactivityin Test Plant Species.
|
Superoxide Dismutase Activity (U g-1 FW) in Plant Parts |
||||||||||||||
|
Plant species |
Dose (mg kg-1soil) |
Aboveground |
Belowground |
|||||||||||
|
|
30 d |
60d |
30 d |
60d |
||||||||||
|
|
|
|||||||||||||
|
C. indica |
Control |
4.82 ± 0.24 |
5.19 ± 0.21 |
8.99 ± 0.34 |
9.25 ± 0.12 |
|||||||||
|
Cr250 |
8.86 ± 0.15*** |
5.94 ± 0.13*** |
10.10 ± 0.36 |
9.18 ± 0.33 |
||||||||||
|
Cr500 |
6.05 ± 0.28 |
4.61 ± .18 |
7.26 ± 0.60** |
5.63 ± 0.24*** |
||||||||||
|
Cr750 |
2.89 ± 0.54*** |
2.06 ± 0.19*** |
4.12 ± 0.48*** |
3.27 ± 0.18*** |
||||||||||
|
|
|
|
|
|
|
|
||||||||
|
N. exaltata |
Control |
9.80±0.05 |
9.95 ± 0.23 |
6.28 ± 0.62 |
6.56±0.06 |
|||||||||
|
Cr250 |
10.49 ± 0.19 |
10.00 ± 0.23 |
11.67 ± 0.13*** |
8.95 ± 0.35*** |
||||||||||
|
Cr500 |
8.67 ± 0.23** |
8.15 ± 0.31*** |
8.99 ± 0.34*** |
8.69 ± 0.32*** |
||||||||||
|
Cr750 |
6.22 ± 0.41*** |
4.81± 0.46*** |
7.14± 0.18 |
4.82 ± 0.25*** |
||||||||||
|
|
|
|
|
|
|
|
||||||||
|
S. trifasciata |
Control |
5.51 ± 0.33 |
5.52±0.51 |
7.07 ± .40 |
7.80 ± 0.83 |
|||||||||
|
Cr250 |
7.45 ± 0.25*** |
6.44 ± 0.07* |
8.70 ± 0.28*** |
7.48 ± 0.32 |
||||||||||
|
Cr500 |
5.82 ± 0.12 |
5.06 ± 0.41 |
7.72± 0.15 |
6.00 ± 0.10** |
||||||||||
|
Cr750 |
4.35 ± 1.51*** |
3.14 ± 1.29*** |
5.54 ± 0.10*** |
4.16 ± 0.19*** |
||||||||||
|
|
|
|
|
|
|
|||||||||
Values are mean ± standard deviation (n=3), significant difference w.r.t control is represented as * p < 0.05,**p<0.01,***p<0.001
But, at higher concentrations Cr750, there was a significant decrease in the activity of Superoxide dismutase(p<0.05) compared to control in all the species showing 43-60% decline in aboveground and 27-64% decline in belowground parts, as compared to their control. A boost in superoxide dismutase activity in aboveground parts in response to a mild metal stress (Cr250) indicates its likely role in stress tolerance at low Cr concentration.
Metal Accumulation
The plants were investigated for their Cr phytoremediation capability by testing accumulation of themetal in aboveground and belowground parts, separately (Table 5). Uptake of the metal progressively increased in the test plants with increase in concentration and duration of exposure. There was significantly higher concentration of the metal in both above and belowground parts of each species (p<0.05)as compared to respective controls all through.
Table 5: Metal content in the Three Ornamental Species after Metal Exposure.
|
Cr Concentration(µg g-1drywt.) in Plant Parts |
|||||||||||||||
|
Plant species |
Dose (mg kg-1soil) |
Aboveground |
Belowground |
||||||||||||
|
|
|
30d |
60d |
30d |
60d |
||||||||||
|
|
|
|
|
|
|
||||||||||
|
|
|
||||||||||||||
|
C. indica |
Control |
9.54 ± 0.23 |
9.92 ± 0.26 |
18.52 ± 0.26 |
18.55 ± 0.18 |
||||||||||
|
Cr250 |
141.31 ± 2.39*** |
217.13± 16.95*** |
674.87± 8.98*** |
916.24 ± 3.00 *** |
|||||||||||
|
Cr500 |
224.31 ± 3.72*** |
331.67± 11.16*** |
1144.5±37.36*** |
1351.07 ± 31.4*** |
|||||||||||
|
Cr750 |
254.03 ± 25.76*** |
556.7 ± 26.44*** |
1446.6±37.72*** |
1847.4±32.46*** |
|||||||||||
|
|
|||||||||||||||
|
N. exaltata |
Control |
5.18 ± 0.15 |
5.24 ± 0.23 |
12.65 ± 0.12 |
12.76 ± 0.12 |
||||||||||
|
Cr250 |
130.63 ± 16.92*** |
276.47± 53.18*** |
475.5±23.19*** |
654.27±32.08*** |
|||||||||||
|
Cr500 |
218.4 ± 7.11*** |
366.7 ± 55.1*** |
826.1±11.35*** |
1093.9 ± 50.8*** |
|||||||||||
|
Cr750 |
458.33 ± 35.5*** |
607.07± 85.23*** |
1131.9±110**** |
2136.07 ± 140*** |
|||||||||||
|
|
|||||||||||||||
|
S. trifasciata |
Control |
18.62 ± 0.36 |
18.82 ± 0.3 |
14.77 ± 0.08 |
14.83 ± 0.12 |
||||||||||
|
Cr250 |
71.79 ± 5.71*** |
124.8 ± 16.38*** |
222.9 ± 25.7*** |
367.3 ± 17.88*** |
|||||||||||
|
Cr500 |
176.6 ± 10.63*** |
231.49 ± 10.2*** |
332.8 ± 35.8*** |
601.55 ± 131.6*** |
|||||||||||
|
Cr750 |
203.87 ± 8.5*** |
386.1 ± 15.9*** |
569.3 ± 19.3*** |
1101.1 ± 46.2*** |
|||||||||||
|
|
|
|
|
|
|
||||||||||
Values are mean ± standard deviation (n=3), significant difference w.r.t control is represented as * p < 0.05,**p<0.01,***p<0.001
In C.indica, Crconcentrations in the belowground parts were 675- 1848 µg g-1, while that in abovegroundparts were 141- 557 µg g-1. Total Cr accumulation in N.exaltata ranged from 475 to 2136 µg g-1 in belowground and 130 to 607 µg g-1 in aboveground parts. In S.trifasciata, Cr was accumulated in the range of 223-1101 µg g-1 in belowground parts and 72- 386 µg g-1 in aboveground parts. Thus, highest accumulation of Cr was recorded in belowground part of N.exaltata at Cr 750 (60 d).
Phytoextraction Capacity of Test Plant Species
The phytoextraction capacity differed significantly (p<0.001) among plant species and the metal concentrations in the soil. Total Cr extracted was significantly more in belowground parts than in the aboveground parts of Canna and Nephrolepis (Table 6). N. exaltata plants had the highest phytoextraction (20.5 mg) in its belowground parts (60d). Though C. indica also showed good phytoextraction of Cr in its belowground biomass (15.5mg) on 30d, it declined with time. This was because the overall belowground biomass decreased in this species at 60d, though metal concentration was high. On the other hand, S.trifasciata showed almost comparable extraction of the metal in aboveground parts (5.08mg) and belowground parts (4.7mg) at Cr 750 indicating that the plants tend to translocate a balanced proportion of the metal taken up by the roots to the shoots. The overall Cr phytoextraction by these species was up to 24, 17.7 and 9.2mg per plant in Nephrolepis, Canna and Sansevieria, respectively.
Table 6: Phytoextraction Capacity for Cr (mg Plant-1) in Test Plant Species.
|
Phytoextraction Capacity (mg plant-1) |
||||||||||||||||||||
|
Plant species |
Dose (mg kg-1soil) |
Aboveground |
Belowground |
|||||||||||||||||
|
|
30 d |
60d |
30 d |
60d |
||||||||||||||||
|
|
|
|||||||||||||||||||
|
C. indica |
Control |
0.14 ± 0.01 |
0.195 ± 0.01 |
0.39 ± 0.05 |
0.47 ± 0.05 |
|||||||||||||||
|
Cr250 |
1.90 ± 0.07*** |
1.88 ± 0.28*** |
10.80 ± 0.65*** |
11.41 ± 0.36*** |
||||||||||||||||
|
Cr500 |
1.70± 1.35*** |
2.06 ± 0.33*** |
15.51 ± 0.71*** |
11.53 ± 0.85*** |
||||||||||||||||
|
Cr750 |
1.12 ± 0.84*** |
1.45 ± 0.29** |
11.44 ± 1.23*** |
7.82 ± 0.22*** |
||||||||||||||||
|
|
||||||||||||||||||||
|
N. exaltata |
Control |
0.08 ± 0.01 |
0.10 ± 0.01 |
0.2217 ± 0.03 |
0.275 ± 0.01 |
|||||||||||||||
|
Cr250 |
2.05 ± 0.33*** |
3.80 ± 0.78*** |
8.62 ± 0.55*** |
9.64 ± 0.24*** |
||||||||||||||||
|
Cr500 |
2.66 ± 0.11*** |
3.74 ± 0.60*** |
12.67 ± 0.38*** |
14.71 ± 1.16*** |
||||||||||||||||
|
Cr750 |
3.89 ± 0.48*** |
3.77 ± 0.44*** |
13.17 ± 1.28*** |
20.50 ± 2.06*** |
||||||||||||||||
|
|
|
|||||||||||||||||||
|
S.trifasciata |
Control |
0.48 ± 0.03 |
0.51 ± 0.02 |
0.24 ± 0.01 |
0.28 ± 0.01 |
|||||||||||||||
|
Cr250 |
1.89 ± 0.19*** |
2.87 ± 0.44*** |
2.52 ± 0.50*** |
3.38 ± 0.37*** |
||||||||||||||||
|
Cr500 |
4.21 ± 0.49*** |
4.41± 0.21*** |
3.35 ± 0.37*** |
3.99 ± 0.74*** |
||||||||||||||||
|
Cr750 |
3.50± 0.15*** |
5.08 ± 0.16*** |
4.73 ± 0.20*** |
4.17 ± 0.93*** |
||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
Values are mean ± standard deviation (n=3), significant difference from control is represented as * p <0.05, **p<0.01,***p<0.001
Chromium Accumulation Factors
Bioaccumulation and bioconcentration factors for the metal in the three species are shown in Table 7. Bioaccumulation factor (BAF) indicates the ability of the plants to tolerate and accumulate metals in the aboveground parts in relation to the metal concentration in soil14. BAF in all the test species was > 1 except for N.exaltata(1.07) at Cr250 after 60d. BAF for N. exaltata lies in the range from 0.14-1.07, for C.indica it is 0.55 to 0.84 and for S.trifasciata it is 0.27 to 0.50. The BAF values tended to increase as Cr concentrations in soil increased, but remained < 1, indicating that none of these speciesact as metal accumulator. BAF values show that these plant species have lesser tendency to accumulate Cr in aerial parts of the plants indicating low translocation.
Table 7: Chromium Accumulation Factors (BAF and BCF) in the Plant Species.
|
Accumulation Factors |
||||||||||||||||||||
|
Plant species |
Dose (mg kg-1soil) |
Bioaccumulation factor |
Bioconcentration factor |
|||||||||||||||||
|
|
30d |
60d |
30d |
60d |
||||||||||||||||
|
|
||||||||||||||||||||
|
C. indica |
Control |
0.25 ± 0.01 |
0.26 ± 0.01 |
0.48 ± 0.01 |
0.49± 0.01 |
|||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
|
Cr250 |
0.55 ± 0.01*** |
0.84 ± 0.07*** |
2.62 ± 0.03*** |
3.54 ± 0.01*** |
||||||||||||||||
|
Cr500 |
0.43 ± 0.01*** |
0.64 ± 0.02*** |
2.21 ± 0.07*** |
2.61 ± 0.06*** |
||||||||||||||||
|
Cr750 |
0.33 ± 0.03** |
0.72 ± 0.03*** |
1.89 ± 0.04*** |
2.40 ± 0.04*** |
||||||||||||||||
|
|
||||||||||||||||||||
|
N. exaltata |
Control |
0.14 ± 0.01 |
0.14 ± 0.01 |
0.33 ± 0.01 |
0.33 ± 0.01 |
|||||||||||||||
|
Cr250 |
0.51 ± 0.07*** |
1.07 ± 0.21*** |
1.84 ± 0.09*** |
2.54 ± 0.12*** |
||||||||||||||||
|
Cr500 |
0.42 ± 0.01*** |
0.71 ± 0.11** |
1.60 ± 0.02*** |
2.12 ± 0.1*** |
||||||||||||||||
|
Cr750 |
0.60 ± 0.05*** |
0.79 ± 0.11*** |
1.47 ± 0.14*** |
2.78 ± 0.15*** |
||||||||||||||||
|
|
||||||||||||||||||||
|
S. trifasciata |
Control |
0.49 ± 0.01 |
0.49 ± 0.01 |
0.39 ± 0.01 |
0.39 ± 0.01 |
|||||||||||||||
|
Cr250 |
0.29 ± 0.02*** |
0.48 ± 0.06 |
0.86± 0.10*** |
1.42 ± 0.07*** |
||||||||||||||||
|
Cr500 |
0.34 ± 0.02*** |
0.45 ± 0.02 |
0.64 ± 0.07** |
1.16 ± 0.26*** |
||||||||||||||||
|
Cr750 |
0.27 ± 0.01*** |
0.50 ± 0.02 |
0.74 ± 0.03*** |
1.43 ± 0.06*** |
||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
Values are mean ± standard deviation (n=3), significant difference w.r.t control is represented as * p < 0.05,**p<0.01,***p<0.001
Bioconcentration factor (BCF) indicating metal accumulation in belowground parts of the plants in relation to the concentration of the metal in soil Yoon 15showed that all the three species had a potential for phytoremediation. The values of BCF for C.indica is much higher (1.89 to 3.54) as compared to that of S.trifasciata (0.64 to 1.16) and N.exaltata ( 1.47 to 2.78) as may be seen in Table7. BCF values >1 indicate good phytoremediation potential of plants at different concentrations of chromium. Bioconcentration factor (BCF) values between 0.1 to 1 indicates that the plant species is a moderate accumulator, while that with BCF value greater than one suggests that it is metal accumulator. Thus, all the species have potential for bioremediation of Cr by concentrating the metal in belowground parts.
Discussion
Plant species in the present study, though showed a significant decline in aboveground biomass at higher Cr concentrations on 60d, and in belowground biomass all through, yet it is noteworthy that the plants continued to grow and thrive even under the harsh metal contaminated conditions. In the present study, Nephrolepis showed good biomass of belowground parts while Sansevieria showed high aboveground biomass in the presence of the metal.
Presence of Cr in concentrations greater than 250 mg kg -1 in the soil had significant impact on major photosynthetic pigments like Chlorophyll a, b, total chlorophyll and carotenoids in all the plants , while lower concentrations had no significant effect on the pigments. However, all plants showed early symptoms of senescence when exposed to the metal, thereby showing some loss of chlorophyll at 60 d of sampling. Thylakoidmembranes of chloroplasts are reported be break down due to Cr toxicity resulting in decrease in chlorophyll level in plants17. Decrease in chlorophyll a and b content in Brassica oleracea (L.), a plant used in bioremediation, was reported by Ozdener 18on Cr exposure. Several studies show that Cr stress leads to oxidative stress and a decrease in total chlorophyll, chlorophyll a and b 19,20. Though Cr is known to have toxic effects on plant chlorophyll, but a slight promoting effect on chlorophyll has also been reported sometimes21, as also in case of Sansevieria in the present study. Since successful growth of a plant species is governed largely by its photosynthetic pigments, hence effect of the metal on these pigments is important. Photosynthetic pigments in the test plant species were quite resistant to Cr, and even increased under low concentrations (< 250 mg kg -1), which suggests suitability of their use in bioremediation.
Metals are known to cause oxidative stress in plants and there are some enzymes that have anti-oxidative action to protect the cells from damage. In metal stressed plants antioxidant enzyme activities are highly variable and depend on various factors like plant species, metal concentration and its duration of exposure 22. Amongst antioxidant enzymes, catalase activity which has potential to scavenge H2O2 was found to decline in both time as well as dose dependent manner in all the test plant species. Though this enzyme is often reported to help against the oxidative stress induced by heavy metals, reduced CAT activity has been reported in different plant species exposed to chromium 23, 24. No direct role of catalase was thus observed in combating the oxidative stress caused by chromium at higher concentration in the test species. However, superoxide dismutase (SOD) which is the first enzyme in the detoxification process of free radicles showed increased activity in both aboveground and belowground parts of plants in all test plant species at lower concentrations of Cr. An elevated level of SOD shows an active antioxidant defense system. A boost in superoxide dismutase activity in response to a mild metal stress (Cr250) indicates its role in stress tolerance. Several studies have shown earlier an increase in superoxide dismutase activity in higher plants due to oxidative stress induced by Cr 25. Decreased superoxide dismutase activity at Cr750 in the test species could be due to excess production of reactive oxygen species in the presence of high concentration of the metal, as suggested by Dazy 23. Decline in SOD activity with increase in concentration of heavy metals in comparison to their lower concentration has been reported earlier by Sinha26. The results show that none of the three ornamental species studied here have a robust anti-oxidative defense enzyme against Cr toxicity especially at 750 ppm concentration.
Phytoextraction capacity of the test plant species was calculated to know the actual uptake of the heavy metal in plant biomass. The phytoextraction was found to be more in belowground parts as compared to aboveground parts of Nephrolepis and Canna and the reverse in Sansevieria, which had overall lesser phytoextraction capability for the metal. Cr accumulation factor for aboveground parts was found to be less than 1, both for Sansevieria and Cannaindicating low transfer of the metal to shoots. Low translocation of Cr to metabolically more active aboveground parts of all test plants at high Cr concentration (Cr750) indicates restricted transportation of Cr to aerial parts.
The test species responded in a dosedependent as well as time dependent mannershowing maximum accumulation of Cr (60 d) when the soil metal concentration was 750ppm. All the speciesshowed higher Cr concentration in the belowground than in aboveground parts indicating low translocation.Significant accumulation of Cr in belowground parts of plants indicates that these species tend to take up the metal from contaminated soils, but store it mostly in the belowground tissues, which is amechanism of phytostabilization of the metals. High Cr accumulation in roots have been reported earlier by some researchers 27,28and in Sesbania cannabina on treatment with fly ash 29.
Greater accumulation of Cr in roots or rhizomes seem to be helpful for the plantas high levels of the metal in shoots and leaves can interfere with the major metabolic activities of the plant. Immobilization of Cr in the vacuoles of the root cellsis a natural response of some plantsto reduce toxic impacts on the plant24. Since Cr is known to be very toxic in nature, it affects plant metabolism and growth.Reduced plant biomass at high Cr concentration is reported for some oil yielding plant species like Brassica and Jatropha30,31. For uptake of metal ions, the plants need to spend extra energy because of which there is decrease in plant biomass with increasing concentration of Cr32.
Similar findings were reported by other researchers with higher BCF in roots than shoots for Cr metal in Sesbania virgate 33. Similar findings of poor translocation of Cr from root to shoots have been reported in aquatic plant Eichhornia crassipes. Lytle 34 reported that Cr (VI), which is a more toxic form of the metal gets reduced to Cr(III) and is retained in roots some tolerant plant species.Thus, the metal is phytostabilized in the belowground plant parts in a less toxic form. Enzymes such as Fe (III) reductase are known to reduce Cr(VI) to Cr(III) in belowground parts35. Phytoextraction capacity, indicating efficiency of a species for heavy metal removal from the soil, depends upon the metal concentration in the plant and its biomass36.
Bioconcentration factor (BCF) between 0.1 to1.0 indicates that the plant species is a moderate accumulator, while plants with BCFvalues >1 suggests that it is metal accumulator 37,38. In the present study, all the three species show BCF >1 suggesting that these can be used for phytoremediation of Cr from soil. Canna indica andNephrolepis exaltata show excellent phytoremediation potential. The tendency of these plant species to concentrate Cr in the belowground parts(rhizome or roots) and limited translocation to aerial parts is of great advantage, as there would be lesser chances of movement of the toxic metal through herbivory.
Conclusion
All three test plant species (S.trifasciata,N.exaltata and C. indica) were found to take up Cr from the contaminated soils and accumulate. The plantspecies showedexcellent tolerance to low Cr pollutionin terms of biomass and photosynthetic pigments. High superoxide dismutase activity in all the three plant species up to 500ppm concentration of the metal suggested that anti-oxidative action of this enzyme helps combat the oxidative stress caused by the metal. These herbaceous perennial ornamental plantscouldsuccessfully grow in the contaminated soil, remove the toxic metal,and accumulate the same mainly in the belowground parts,with restricted translocation to aerial parts, except that inS.trifasciata, which had relativelymore Cr accumulation in aboveground parts. Possibilities of transferof Cr through insect herbivory and food chain are reduced when the metal accumulation is mainly in belowground parts. Use of ornamental plant species has thus a great potential to for sustainable phytoremediationof chromium contaminated soils in aesthetic way.
Acknowledgments
The authors would like to thank GGS Indraprastha University, Delhifor providing the infra-structure facilities including the Greenhouse where all pot experiments were conducted.
Funding Source
The authors would like to thank GGS Indraprastha University, Delhi for student fellowship (STRF) to GS and Faculty Research Grant to AK for the present study.
References
- Baral A, Engelken R, Stephens W, Farris J, Hannigan R. Evaluation of Aquatic Toxicities of Chromium and Chromium-Containing Effluents in Reference to Chromium Electroplating Industries. Arch Environ Contam Toxicol. 2006;50(4):496-502.
CrossRef - Bielicka A, Bojanowska I, Wisniewski A. Two Faces of Chromium-Pollutant and Bioelement. Polish J.Environ.Stud.2005 :14(1):5-10
- Kabata-Pendias, A, Pendias, H. Trace elements in plants and soils. CRC press.Boca Raton, Florida: 1984:233-237.
- Lone M, He Z, Stoffella P, Yang X. Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. J. Zhejiang University Sci. B. 2008;9(3):210-220.
CrossRef - Feng Q, Tai P, Li P, Guo Y, Fu S. Role of Sulfur in Cadmium Accumulation ofTagetes erectaL. J Plant Nutr. 2009;32(6):919-928.
CrossRef - Marques A, Moreira H, Franco A, Rangel A, Castro P. Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria – Effects on phytoremediation strategies. Chemosphere. 2013;92(1):74-83.
CrossRef - Sehrawat G, Kaushik A, Singh R. Ornamental Plant Species for Application in Phytoremediation of Metal Contaminated Soils. Environ. We Int. J. Sci. Tech.2021:16:15-23.
- Arnon D. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1-15.
CrossRef - Lichtenthaler H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth Enzymol.1987: 148:350-382.
CrossRef - Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46(2):849-854.
CrossRef - Kakkar P, Das B, Viswanathan,P,. A modified spectro-photometric assay of superoxide dismutase. Ind. J. Biochem.Biophys.1984:21: 131–132
- Sinha A. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389-394.
CrossRef - Sahu R, Katiyar S, Tiwari J, Kisku G. Assessment of drain water receiving effluent from tanneries and its impact on soil and plants with particular emphasis on bioaccumulation of heavy metals. J. Environ. Biol.2007;28(3):685–690
- Caille N, Zhao F, McGrath S. Comparison of root absorption, translocation and tolerance of arsenic in the hyperaccumulator Pteris vittata and the nonhyperaccumulator Pteris tremula. New Phytologist. 2004;165(3):755-761.
CrossRef - Yoon J, Cao X, Zhou Q, Ma L. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006; 368(2-3):456-464.
CrossRef - Allen S, Grimshaw, H, Rowland A. Chemical analysis. In: Moore P, Chapman S,ed. Methods In Plant Ecology.. Oxford: Blackwell Scientific Publications.; 1986:285-344.
- Dodge J,Lawes G.Plastid ultrastructure in some parasitic and semi-parasitic plants. Cytobiology .1974: 9: 1–9
- Ozdener Y, Aydin B, Fatma Aygün S, Yürekli F. Effect of hexavalent chromium on the growth and physiological and biochemical parameters on Brassica oleracea L. var.acephala DC. Acta Biol Hung. 2011;62(4):463-476.
CrossRef - Panda S, Khan M .Antioxidant efficiency in rice (Oryza sativa L.) leaves under heavy metal toxicity. J Plant Biol .2003: 30(1):23–29
- Hamid R, Parray JA, Kamili AN, Mahmooduzzafar. Chromium stress in Brassica juncea L. cv. 'Pusa Jai Kissan' under hydroponic culture. Afric J Biotech. 2012: 11(90):15658–15663.
CrossRef - Pandey V, Dixit V, Shyam R (2005) Antioxidative responses in relation to growth of mustard (Brassica juncea cv. Pusa Jaikisan) plants exposed to hexavalent chromium. Chemosphere 61:40–47.
CrossRef - Sharma S, Dietz K. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009;14(1):43-50.
CrossRef - Dazy M, Béraud E, Cotelle S, Meux E, Masfaraud J, Férard J. Antioxidant enzyme activities as affected by trivalent and hexavalent chromium species in Fontinalis antipyretica Hedw. Chemosphere. 2008;73(3):281-290.
CrossRef - Shanker A, Cervantes C, Lozatavera H, Avudainayagam S. Chromium toxicity in plants. Environ Int. 2005;31(5):739-753.
CrossRef - Karuppanapandian, T, Sinha PB, Haniya A, Mamoharan K. Differential antioxidative responses of ascorbate-glutathione cycle enzymes and metabolites to chromium stress in green gram (Vigna radiata L. Wilczek) leaves. J. Plant.Biol.2006:49(6):440-447.
CrossRef - Sinha S, Sinam G, Mishra R, Mallick S. Metal accumulation, growth, antioxidants and oil yield of Brassica juncea L. exposed to different metals. Ecotoxicol Environ Saf. 2010; 73(6):1352-1361.
CrossRef - Shanker A, Pathmanabhan G. Speciation dependant antioxidative response in roots and leaves of sorghum (Sorghum bicolor (L.) Moench cv CO 27) under Cr(III) and Cr(VI) stress. Plant Soil. 2004;265(1-2):141-151.
CrossRef - Yadav S, Dhote M, Kumar P, Sharma J, Chakrabarti T, Juwarkar A. Differential antioxidative enzyme responses of Jatropha curcas L. to chromium stress. J Hazard Mater. 2010;180(1-3):609-615.
CrossRef - Sinha S, Gupta A. Translocation of metals from fly ash amended soil in the plant of Sesbania cannabina L. Ritz: Effect on antioxidants. Chemosphere. 2005;61(8):1204-1214.
CrossRef - Diwan H, Ahmad A, Iqbal M. Chromium-Induced Modulation in the Antioxidant Defense System During Phenological Growth Stages of Indian Mustard .Int J Phytoremediation. 2009;12(2):142-158.
CrossRef - Yadav S, Juwarkar A, Kumar G, Thawale P, Singh S, Chakrabarti T. Bioaccumulation and phyto-translocation of arsenic, chromium and zinc by Jatropha curcas L.: Impact of dairy sludge and biofertilizer. Bioresour Technol. 2009;100(20):4616-4622.
CrossRef - Shanker,A, Djanaguiraman M., Sudhagar R., Chandrashekar, C, Pathmanabhan, G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R. Wilczek. cv CO 4) roots. Plant Sci, 2004. 166(4): 1035-1043.
CrossRef - Branzini A, González R, Zubillaga M. Absorption and translocation of copper, zinc and chromium by Sesbania virgata. J Environ Manage. 2012;102:50-54.
CrossRef - Lytle C, Lytle F, Yang N et al. Reduction of Cr(VI) to Cr(III) by Wetland Plants: Potential for In Situ Heavy Metal Detoxification. Environ Sci Technol. 1998;32(20):3087-3093.
CrossRef - Zayed A, Lytle C, Qian J, Terry N. Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta. 1998;206(2):293-299.
CrossRef - Kachenko A, Singh B, Bhatia N. Heavy metal tolerance in common fern species. Aust. J.Bot. 2007;55(1):63-73.
CrossRef - Baker A. Accumulators and excluders?strategies in the response of plants to heavy metals. J Plant Nutr. 1981;3(1-4):643-654.
CrossRef - Ma L, Komar K, Tu C, Zhang W, Cai Y, Kennelley E. A fern that hyperaccumulates arsenic. Nature. 2001;409(6820):579-579.
CrossRef







