Pretreatment, Hydrolysis and Fermentation of Lignocellulosic Biomass for Bioethanol
1
Department of Chemical Engineering,
Osmania University,
Hyderabad,
India
Corresponding author Email: suddhu.vanam@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.17.1.10
Currently, only bioethanol may be used in fuel systems without requiring significant changes to the fuel distribution system. Furthermore, burning bioethanol creates the same amount of CO2 as the plant produces when growing, therefore it does not contribute to the increase in the greenhouse effect. Biodiesel can be made from plants that produce sugar or plants that contain starch (wheat, corn, etc.). However, producing bioethanol on a large scale necessitates the use of vast swaths of land for maize or sugarcane farming. Lignocellulosic biomass, such as agricultural leftovers, may be a solution to this problem, despite technical issues, due to its great availability and low cost. In this article, we will go over the many methods for pretreatment of lignocellulosic biomass, as well as the several fermentation procedures that can be used to get bioethanol from it.
Copy the following to cite this article:
Sudhakar V, Naik S. S. Pretreatment, Hydrolysis and Fermentation of Lignocellulosic Biomass for Bioethanol.Curr World Environ 2022;17(1). DOI:http://dx.doi.org/10.12944/CWE.17.1.10
Copy the following to cite this URL:
Sudhakar V, Naik S. S. Pretreatment, Hydrolysis and Fermentation of Lignocellulosic Biomass for Bioethanol.Curr World Environ 2022;17(1).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 15-08-2021 |
|---|---|
| Accepted: | 22-12-2021 |
| Reviewed by: | 
 Aparna Gunjal
Aparna Gunjal
|
| Second Review by: |

 Roshan Kumar
Roshan Kumar
|
| Final Approval by: | Dr. Marta Luciane Fischer |
Introduction
Oil productioniscurrently the world's most important source of primary energy use. Because of the growth of emerging countries' transportation and manufacturing sectors, oil demand is expected to jump from 60% to 75% by 2030. Furthermore, using fossil fuels increases greenhouse gas emissions, particularly carbon dioxide (CO2). These greenhouse gases are to blame for the earth's global warming and the current climatic deregulation 1.As a result, in a global setting defined by the volatility of oil barrel prices and in the interests of environmental conservation, new and renewable energy is being produced. Biofuels are one of these energy sources that is gaining popularity around the world 2 because they can be used in combustion engines. The European Commission has established a precedent in this area by committing to a 20 percent renewable energy target in total energy consumption by 2020, with biofuels expected to play a vital role in achieving this goal 3. The most abundant renewable carbon source on the planet is lignocellulosic biomass (LCB) 4. It is made up largely of cellulose, hemicellulose, and lignin in varied quantities and comes in a variety of forms (agricultural leftovers, forest wastes, etc.). Lignocellulosic biomass also contains polysaccharides that can be converted into simple fermentable sugars for the production of biofuels such as second-generation bioethanol 5.Unlike first-generation bioethanol, which is frequently made from sugar and amylaceous plants and raises ethical concerns owing to the use of foodstuffs, second-generation bioethanol is entirely made from plant components and does not compete with food production. Second-generation bioethanol development is now widely recognized as the most promising biofuels business, with many governments throughout the world taking an interest 6.One of the recommended techniques for the synthesis of these biofuels is the biochemical process, which uses enzymes and microorganisms to convert Lignocellulosic biomass into ethanol. However, because the output from this process is modest, process optimization and control are crucial for the sector's economic viability. Lignocellulosic biomass is cellulose-based biomass that is both affordable and readily available for energy production. Agricultural residues, energy crops, forest residues, and cellulosic wastes are all excellent sources of lignocellulosic biomass. The global lignocellulosic biomass production is 181.5 billion tonnes per year 7. Lignocellulosic biomass energy accounts for around 10% of global energy demand 9. Agricultural and forest leftovers alone contributed 30 EJ, which is a significant amount, to the yearly energy use of 4500 EJ 9. Biofuels, particularly bioethanol, bio-oil, gasoline, and chemicals can all be made from lignocellulosic resources. For lignocellulosic biomass conversion, different types of conversion technologies exist, including thermal, thermochemical, and biological methods. Bioconversion is required to turn biomass into biofuels using microorganisms. Its major product is bioethanol, although it also makes biobutanol, methane, and a few other compounds 10.Pre-treatments using chemicals and physio chemicals are now the most successful. However, because they are unfriendly to the environment and produce hazardous compounds like furfural, eco-friendly biological pre-treatment procedures are occasionally applied 11. It is a requirement for promoting cost-effective sustainable energy. However, implementing them on a commercial scale is difficult 12. Recent research on lignin chemistry and valorisation has been published 13,14. This research looks at the various Lignocellulosic biomass pretreatment methods as well as the several lignocellulosic fermentation procedures that have recently been created to boost ethanol output via lignocellulosic fermentation.
Lignocellulosic Biomass to Bioethanol
To convert lignocellulosic biomass into bioethanol, the biomass must be pretreated, hydrolyzed, and fermented. Lignin is removed or modified, hemicellulose is extracted, cellulose is crystallized, the acetyl group is removed from hemicellulose, Cellulose polymerization is reduced to expand the structure, pore values ??and internal surface area are increased, and hydrolysis of the carbohydrate fraction per monomer may occur faster and in higher yields 15. According to several studies 16, 17, different pretreatment procedures have distinct effects on biomass. There must be pretreatment to disrupt or eliminate lignin from lignocellulosic biomass in order to improve accessibility to cellulose 18, 19. The expense and effort involved in many preparation operations, on the other hand, can be substantial. The compatibility of the conversion process has also been found to be affected by various delignification processes 20. It is difficult to hydrolyze cellulase enzyme if the pretreatment is inadequate, and toxic byproducts are created if the pretreatment is more severe, reducing the growth of fermentative microbial strains and decreasing bioethanol output 21. Adapted figure.1 22 illustrates the goal of lignocellulosic biomass pretreatment by removing lignin by releasing cellulose and hemicellulose from the biomass. To improve the accessibility of cellulosic materials for enzymatic hydrolysis, several pretreatment techniques have been devised. Pretreatment can be classified in 15 distinct ways, including mechanical, physio-chemical, chemical, and biological. Only a few of these techniques, however, have received sufficient development to be applied in industrial settings 24.
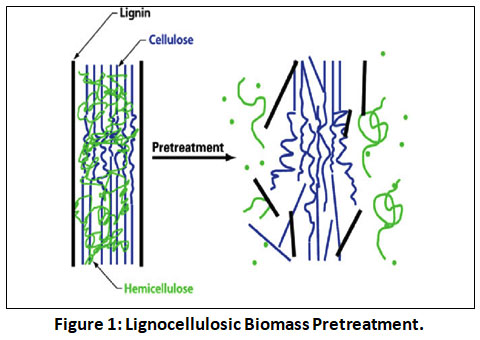 | 1: Lignocellulosic Biomass Pretreatment.Figure Click here to view Figure |
Mechanical pre-treatment
Mechanical pre-treatment involves reducing biomass particle size to promote surface accessibility and speed up hydrolysis. The majority of lignocellulosic material is crushed down to particles less than 2 mm in size. However, while research on micronized substrates yields better results than crushed substrates, testing on micronized substrates offer greater outcomes. Micronization is essential to produce a significant increase in yield. After 24 hours of hydrolysis, the percentage yield of each glucose and xylose was enhanced from 53 to 149 m by pre-treatment to a 53–149 m particle size and compared to a powdered substrate with a particle size of 2–4 cm. Enzymatic hydrolysis produced 61.1 percent of the micronized material in research by Talebnia et al. [26], but only 17.1 percent of the non- pre-treated sample. Finally, the above treatment does not produce furfuraldealdehyde, which is a yeast inhibitor that inhibits subsequent fermentation. When hemicelluloses and phenolic compounds, both of which are generated during the degradation of lignin, are destroyed, the substance is formed.
Physico-chemical pre-treatment
Combine physical and chemical treatments that rearrange lignin structures to increase the accessibility of cellulose for hydrolysis to fully recover hemicellulose. There is a lot of interest in leveraging steam explosion's physical and chemical processes to make biomass easier to access for hydrolysis [30]. The material is heated to over 300 degrees Fahrenheit [31] and then allowed to cool using this method. The crystallinity of cellulose is increased by steam explosion pretreatment, and the amorphous portions are therefore depolymerized, making hemicellulose hydrolysis and delignification much easier. This process is safer for the environment, less harmful, and yields more sugar. In order to obtain higher bioethanol production from agricultural waste, steam explosion technology is used as a pretreatment process, followed by an alkaline bleaching process. Additional H2SO4 (or SO2) or CO2 can be added to boost the hydrolysis of lignocellulosic waste in a steam explosion, resulting in effective breakdown of complex polysaccharides, a reduction in hazardous by-products, and complete liquefaction of glucan, xylan, mannan, galactan, and arabinan. Ammonia fibre explosion (AFEX) is a fundamental alkaline pretreatment procedure in which liquid ammonia is introduced under high pressure and rapidly decompressed. Biomass with lower amounts of lignin and hemicellulose is more efficient in AFEX, which implies it can avoid increasing hemicellulose solubility by avoiding pretreatment methods such dilute acid pretreatment. In the event of a CO2 explosion, a release of 75% potential glucose was recorded after 24 hours of enzymatic hydrolysis [35]. The physio-chemical treatment of lignocellulosic waste results in a theoretical yield of 83 percent, resulting in a maximum bioethanol yield of 83 percent.
Chemical pre-treatment
Among the most prominent chemical pretreatment treatments are acid, alkaline and ammonia, as well as sulphite, sodium chlorite and organic and inorganic solvents, as well as SO2 and CO2. Sodium sulphite and/or sodium chlorite can eliminate 90 percent of the lignin. Hemicellulose and cellulose are less enzyme-available following the alkali pretreatment. This includes sodium, potassium, calcium, and ammonium carbonate 15 and ammonium hydroxide as pretreatment chemicals that are allowed. The most study has been done on sodium hydroxide 43. Pretreatment of lignocellulosic biomass with alkaline peroxide was used in manufacturing operations. This technique increases enzymatic hydrolysis by allowing it to occur before delignification 24. Organo-solvent hydrolysis of cellulose is facilitated by the use of treated cellulose. The structure of lignin and hemicellulose can be loosened or destroyed with the use of an aqueous organic solvent. When paired with semi-simultaneous saccharification- fermentation (SSSF), alkaline peroxide pretreatment and SSSF were found to be highly efficient and attractive methods for increasing bioethanol production. Ethanol yield was reported to be around 63.1 percent after pretreatment with 10 percent H2O2 at 160°C for 2 hours, followed by acid reflux. Oxidative lignocellulose degradation is called "ozonolysis." Most of the pretreatment takes place at room temperature, and no inhibitory compounds are produced.
Dilute acid pre-treatment
When turning cellulosic biomass into biofuel, the earliest technique of producing bioethanol is through pretreatment with dilute acid. Shearing is an effective way to increase the amount of saccharification-processing-capable cellulosic biomass 46.It has been stated that pretreatment with dilute acid aids in the efficient enzymatic hydrolysis of cotton gin waste 47,48. However, it has been reported that nitric acid, phosphoric acid, organic acid, and HCl have also been used to pretreat lignocellulosic biomass. Acid treatment can have a deleterious impact on the growth of yeast during the fermentation process. Some of the inhibitors produced as a result of acid treatment are hazardous, and this reduces bioethanol yield. There are also serious negative effects that might occur when large volumes of gypsum are used in pretreatment. Pretreatment of cotton gin waste with organic acid provides specific advantages over other traditional acids. [Traduzione originale] Current research in this subject, notably in the area of cotton gin waste bioethanol production, is lacking. In contrast, temperature management minimises the decomposition of sugar, as explained above. Because of the higher temperatures, pretreatment times must be reduced. Corrosion and associated toxicity to microorganisms make concentrated acids unsuitable for bioethanol fermentation as a pretreatment. When inhibitory compounds are formed, corrosion and toxicity follow. It is necessary to collect the acids after the process has been completed in order to boost the procedure's economic viability Cotton gin waste can be pretreated with dilute acid to enhance enzyme hydrolysis 47,48. It takes two phases to accomplish dilute acid hydrolysis to take advantage of the distinctions between hemicellulose and cellulose. This first step begins with a gentle, essentially unselective treatment of the sugar in order to eliminate the five-carbon sugar molecules. A second stage involves biological or chemical treatment of only the residual solids in order to obtain six-carbon sugars 56.
Detoxification
Hemicellulose depolymerization produces xylose as the major product following pretreatment with HCl, with different pretreatment procedures yielding variable quantities of xylose. Although this method has some drawbacks, it does have certain advantages, such as the ability to create damaging inhibitors 38, 57, 58. Three major families of these hazardous by-products are organic acids, furan derivatives and phenolic compounds. Inhibition of yeast cell physiology causes decreased bioethanol generation and productivity 59, 22. Excessive liming 59, ethyl acetate extraction 60, and activated charcoal adsorption 53 have all been investigated for their ability to assist in the removal of fermentation inhibitory products like laccase oxidation treatment. The most common methods are overliming and activated charcoal adsorption, either alone or in combination. Activated charcoal detoxification of hydrolysates is stated to be both cost-effective and high-capacity, with little influence on hydrolysate or sugar levels 38,59.
Biological pretreatment
White, brown, and soft-rot fungi are commonly utilized in biological pretreatment. They're employed to break down complex lignin and dissolve hemicellulose. While white-rot fungus are the most effective microorganisms for delignification of lignocellulosic biomass, this only applies to wood, not polymers. The employment of fungal strains is the most intriguing of the biotic processes that are most suited for turning this waste into bioethanol. The synthesis of bioethanol from lignocellulosic biomass can be both economical and environmentally friendly. The conventional analysis and corrosion formation procedures, in contrast to the traditional approach, necessitate high temperatures, pressures, and energy. Fungal treatment is a biologic pretreatment that uses enzymes found in live cells to break down lignin and hemicellulose compounds in biomass. It produces few by-products, although it usually results in chemical change. In general, for this biologic pretreatment to be successful, moderate environmental conditions are required. Because they resulted in greater enzyme activity, the majority of mixed cultures of white rot fungus were renowned for their synergistic activities 65, 64. Because of synergistic interactions, fungal combinations have the ability to produce more enzymes, but the results appear to be reliant on a number of factors, including the species combination and interaction style among species, the type of substrate, and the surrounding micro-environment. P. chrysosporium, a member of the holobasidiomycetes, is the best studied of the white-rot fungus. After using fungus treatment on cotton plant waste, Pleurotus sajor caju were tested for lignin breakdown by bacteria. The oyster fungus Pleurotus ostreatus was used to study the biodegradation of cotton stalks and cotton seed hull in order to see if it may increase the amount of bioethanol generated 68. There have been reports that Agrocybe cylindracea and Pleurotus ostreatus produced bioethanol using solid-state fermentation, which has sparked a lot of curiosity. Phenol oxidases are the key enzymes involved in the oxidative degradation of lignin by white rot fungi.
Hydrolysis
Cellulose and hemicellulose are depolymerized into simple fermentable sugars during the hydrolysis phase of bioethanol production. Due to its crystalline structure, cellulose is more difficult to hydrolyze than hemicellulose. As a result, cellulose hydrolysis is always carried out using an acid or specialized enzymes. This is referred to as chemical or enzymatic hydrolysis, while the cellulose breakdown is referred to as biochemical hydrolysis or saccharification.
Fermentation Process
Fermentation is a biological process that transforms simple carbohydrates into smaller compounds such as acids and alcohols, and is catalyzed by enzymes released by microorganisms. The two most common carbohydrates are fermented into ethanol using the following two reactions: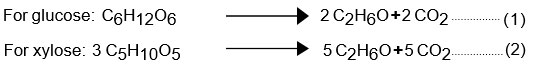
Despite the fact that these sugars are essential for their metabolism and reproduction, they can be fermented by a wide range of bacteria and yeasts. Saccharomyces cerevisiae and Zymomonas mobilis are the two most common species used to make ethanol on a large scale. Saccharomyces cerevisiae is the most extensively used and explored species for the production of bioethanol due to its robustness and appropriateness for the fermentation of glucose from lignocellulosic biomass. Pentose, on the other hand, cannot be fermented via hemicellulose hydrolysis, thus it must be fermented by a different microbe. Z. mobilis, on the other hand, is less extensively used since it has a lower yield and a more active metabolism, making it more susceptible to contamination. Furthermore, alcoholic fermentation necessitates a nutrient-rich media, such as nitrogen, Sulphur, and phosphorus, which influence the cell's ability to endure stress caused by ethanol and/or the nutrients, in addition to serving as a source of cell synthesis. However, keep in mind that physicochemical parameters such as pH and temperature have an impact on fermentation [pH and temperature]. The optimum ethanol production temperature for S. cerevisiae is 33 °C. In ethanol manufacturing, maintaining a pH range of 4.0 to 4.8 is critical 76. Although the ethanol produced during fermentation can be harmful to yeast cells, at a concentration of 7% (V/V), the yeast's inhibitory actions start to show. The Gay-Lussac yield is the theoretical maximum yield of glucose fermentation in ethanol calculated using equation (1). Because this yield excludes sugar losses from biomass production and the conversion of glucose to glycerol and other products, it excludes the final product's potential sugar yield. The highest theoretical production, based on these losses, is calculated to be 0.484 g of ethanol per gram of glucose.
Conclusion
The lignocellulosic bio refinery process would be difficult to execute without pretreatment. Innovative treatments and techniques that result in decreased pretreatment costs, the generation of less harmful compounds, greater sugar yields, and increased by-product values are currently in high demand. The type of biomass, the value of by-products, and the level of complexity of the process are all factors to consider when choosing a pretreatment method. In the future, different approaches might yield better results.
Acknowledgment
The author would like to thank Osmania University for granting the Ph.D. research work. The Department of Chemical Engineering, University College of technology is highly appreciated for allowed the PG laboratory work.
Conflict of Interest
The authors do not have any conflict of interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Australian Academy of Science. The science of climate change: questions and answers. Canberra: Australian Academy of Science; 2015.
- Bai FW, Anderson WA, Moo-Young M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol Adv. 2008; 26(1):89–105.
CrossRef - Commission E. Report from the commission to the European parliament, the Council, the European economic and social committee and the committee of the regions. Brussels: Commission European; 2015.
- Chang VS, Holtzapple MT. Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol 2000; 84– 86(1–9):5–38.
CrossRef - Jorgensen H, Kristensen JB, Felby C. Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefining 2007; 1(2):119–34.
CrossRef - Moon SK, Kim SW, Choi GW. Simultaneous saccharification and continuous fermentation of sludge-containing mash for bioethanol production by Saccharomyces cerevisiae CHFY0321. J Biotechnol 2012; 157(4):584–9.
CrossRef - Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated Lignocellulosic Value Chains in a Growing Bioeconomy: Status Quo and Perspectives. GCB Bioenergy 2018, 11, gcbb.12586.
CrossRef - Hamaguchi, M.; Kautto, J.; Vakkilainen, E. Effects of Hemicellulose Extraction on the Kraft Pulp Mill Operation and Energy Use: Review and Case Study with Lignin Removal. Chem. Eng. Res. Des. 2013, 91, 1284–1291.
CrossRef - Samuel Dahunsi, O.; Enyinnaya, M. The Bioenergy Potentials of Lignocelluloses. In Energy Conversion Current Technologies and Future Trends; IntechOpen: London, UK, 2019.
CrossRef - Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of Lignocellulosic Biomass Conversion to Renewable Fuels. Biomass Convers. Biore?n. 2014, 4, 157–191.
CrossRef - Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi,A.A.DifferentPretreatment Technologies of Lignocellulosic Biomass for Bioethanol Production: An Overview. Energy 2020, 199, 117457.
CrossRef - Ozdenkçi, K.; De Blasio, C.; Muddassar, H.R.; Melin, K.; Oinas, P.; Koskinen, J.; Sarwar, G.; Järvinen, M. A Novel Biore?nery Integration Concept for Lignocellulosic Biomass. Energy Convers. Manag. 2017, 149, 974–987.
CrossRef - Xu, J.; Li, C.; Dai, L.; Xu, C.; Zhong, Y.; Yu, F.; Si, C. Biomass Fractionation and Lignin Fractionation towards Lignin Valorization. ChemSusChem 2020, 4284–4295.
CrossRef - Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 122784.
CrossRef - G.M. AITA, M. KIM, Pretreatment technologies for the conversion of lignocellulosic materials to bioethanol, in: ACS Symp. Ser., 2010: pp. 117–145.
CrossRef - N. Mosier, C. Wyman, B. Dale, R. Elander, Y.Y. Lee, M. Holtzapple, M. Ladisch, Features of promising technologies for pretreatment of lignocellulosic biomass, Bioresour. Technol. 96 (2005) 673–686. doi:10.1016/j.biortech.2004.06.025.
CrossRef - C.E. Wyman, B.E. Dale, R.T. Elander, M. Holtzapple, M.R. Ladisch, Y.Y. Lee, Coordinated development of leading biomass pretreatment technologies, Bioresour. Technol. 96 (2005) 1959–1966. doi:10.1016/j.biortech.2005.01.010.
CrossRef - P. Kaparaju, M. Serrano, A.B. Thomsen, P. Kongjan, I. Angelidaki, Bioethanol, bio hydrogen and biogas production from wheat straw in a bio refinery concept, Bioresour. Technol. 100 (2009) 2562–2568. doi:10.1016/j.biortech.2008.11.011.
CrossRef - X. Zhao, R. Wu, D. Liu, Production of pulp, ethanol and lignin from sugarcane bagasse by alkali-per acetic acid delignification, Biomass and Bioenergy. 35 (2011) 2874–2882. doi:10.1016/j.biombioe.2011.03.033.
CrossRef - K. Safartalab, F. Dadashian, F. Vahabzadeh, Fed batch enzymatic hydrolysis of cotton and viscose waste fibers to produce ethanol, Univers. J. Chem. 2 (2014) 11– 15.
CrossRef - B. Kodali, R. Pogaku, Pretreatment studies of rice bran for the effective production of cellulase, Electron J Env. Agric Food Chem. 5 (2006) 1253–1264.
- T. Hsu, M. Ladisch, G.T. Tsao, Alcohol from Cellulose, Chemtech. (1980) 315– 319.
- V.B. Agbor, N. Cicek, R. Sparling, A. Berlin, D.B. Levin, Biomass pretreatment: Fundamentals toward application, Biotechnol. Adv. 29 (2011) 675–685. doi:10.1016/j.biotechadv.2011.05.005.
CrossRef - M.J. Taherzadeh, K. Karimi, Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review, Int. J. Mol. Sci. 9 (2008) 1621–1651. Doi: 10.3390/ijms9091621.
CrossRef - Ibrahim HAH. Pretreatment of straw for bioethanol production. Energy Procedia 2012; 14:542–51.
CrossRef - Talebnia F, Karakashev D, Angelidaki I. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis, and fermentation. Bioresour Technol 2010; 101(13):4744–53.
CrossRef - Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 2010; 101(13):4851–61.
CrossRef - Jonsson LJ, Alriksson B, Nilvebrant NO. Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 2013; 6(1):1–10.
CrossRef - A.T.W.M. Hendriks, G. Zeeman, Pretreatments to enhance the digestibility of lignocellulosic biomass, Bioresour. Technol. 100 (2009) 10–18. doi:10.1016/j.biortech.2008.05.027.
CrossRef - Q. Liu, K. Cheng, J. Zhang, J. Li, G. Wang, Statistical optimization of recycled paper enzymatic hydrolysis for simultaneous saccharification and fermentation via central composite design., Appl. Biochem. Biotechnol. 160 (2010) 604–12. Doi: 10.1007/s12010-008-8446-2.
CrossRef - G.Y.S. Mtui, Recent advances in pretreatment of lignocellulosic wastes and production of value added products, African J. Biotechnol. Vol. 8 (2009) 1398–1415. doi:10.1073/pnas.1014862107/-/DCSupplemental.www.pnas.org/cgi/.
- M. Balat, Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review, Energy Convers. Manag. 52 (2011) 858–875. doi:10.1016/j.enconman.2010.08.013.
CrossRef - E. Tomas-Pejo, J.M. Oliva, M. Ballesteros, Realistic approach for full-scale bioethanol production from lignocellulose: A review, J. Sci. Ind. Res. (India). 67 (2008) 874–884.
- W.K. El-Zawawy, M.M. Ibrahim, Y.R. Abdel-Fattah, N.A. Soliman, M.M. Mahmoud, Acid and enzyme hydrolysis to convert pretreated lignocellulosic materials into glucose for ethanol production, Carbohydr. Polym. 84 (2011) 865–871. doi:10.1016/j.carbpol.2010.12.022.
CrossRef - Y. Sun, J. Cheng, Hydrolysis of lignocellulosic materials for ethanol production: A review, Bioresour. Technol. 83 (2002) 1–11. Doi: 10.1016/S0960-8524(01)00212-7.
CrossRef - T. Jeoh, F.A. Agblevor, Characterization, and fermentation of steam exploded cotton gin waste, Biomass and Bioenergy. 21 (2001) 109–120. Doi: 10.1016/S09619534 (01)00028-9.
CrossRef - M. a Neves, T. Kimura, N. Shimizu, M. Nakajima, State of the Art and Future Trends of Bioethanol Production, Dyn. Biochem. Process Biotechnol. Mol. Biol. 1 (2007) 1–14.
- R.C. Kuhad, R. Gupta, Y.P. Khasa, A. Singh, Bioethanol production from Lantana camara (red sage): Pretreatment, saccharification and fermentation, Bioresour. Technol. 101 (2010) 8348–8354. doi:10.1016/j.biortech.2010.06.043.
CrossRef - R. Kumari, K. Pramanik, Bioethanol production from Ipomoea Carnea biomass using a potential hybrid yeast strain, Appl. Biochem. Biotechnol. 171 (2013) 771–785. Doi: 10.1007/s12010-013-0398-5.
CrossRef - M. Han, S.K. Moon, Y. Kim, Y. Kim, B. Chung, G.W. Choi, Bioethanol production from ammonia percolated wheat straw, Biotechnol. Bioprocess Eng. 14 (2009) 606–611. Doi: 10.1007/s12257-008-0320-0.
CrossRef - R.A. Silverstein, Y. Chen, R.R. Sharma-Shivappa, M.D. Boyette, J. Osborne, A comparison of chemical pretreatment methods for improving saccharification of cotton stalks, Bioresour. Technol. 98 (2007) 3000–3011. doi:10.1016/j.biortech.2006.10.022.
CrossRef - R. Kumar, G. Mago, V. Balan, C.E. Wyman, Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies, Bioresour. Technol. 100 (2009) 3948–3962. doi:10.1016/j.biortech.2009.01.075.
CrossRef - R. Sindhu, P. Binod, K.U. Janu, R.K. Sukumaran, A. Pandey, Organ solvent pretreatment and enzymatic hydrolysis of rice straw for the production of bioethanol, World J. Microbial. Biotechnol. 28 (2012) 473–483. Doi: 10.1007/s11274-011-0838-8.
CrossRef - L. Zhang, T. You, L. Zhang, H. Yang, F. Xu, Enhanced ferment ability of poplar by combination of alkaline peroxide pretreatment and semi-simultaneous saccharification and fermentation, Bioresour. Technol. 164 (2014) 292–298.
CrossRef - R. Travaini, M.D.M. Otero, M. Coca, R. Da-Silva, S. Bolado, Sugarcane bagasse ozonolysis pretreatment: Effect on enzymatic digestibility and inhibitory compound formation, Bioresour. Technol. 133 (2013) 332–339. doi:10.1016/j.biortech.2013.01.133.
CrossRef - C. Sanchez, Lignocellulosic residues: Biodegradation and bioconversion by fungi, Biotechnol. Adv. 27 (2009) 185–194. doi:10.1016/j.biotechadv.2008.11.001.
CrossRef - R. Kumari, K. Pramanik, Improved bioethanol production using fusants of saccharomyces cerevisiae and Xylose-fermenting yeasts, Appl. Biochem. Biotechnol. 167 (2012) 873–884. Doi: 10.1007/s12010-012-9705-9.
CrossRef - M. Mahalakshmi, J. Angayarkanni, R. Rajendran, R. Rajesh, others, Bioconversion of cotton waste from textile mills to bioethanol by microbial saccharification and fermentation. Ann. Biol. Res. 2 (2011) 380–388.
- D. Scordia, S.L. Cosentino, J.W. Lee, T.W. Jeffries, Dilute oxalic acid pretreatment for bio refining giant reed (Arundo donax L.), Biomass and Bioenergy. 35 (2011) 3018–3024. doi:10.1016/j.biombioe.2011.03.046.
CrossRef - A.M.J. Kootstra, H.H. Beeftink, E.L. Scott, J.P.M. Sanders, Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw, Biochem. Eng. J. 46 (2009) 126–131. doi:10.1016/j.bej.2009.04.020.
CrossRef - L. Qin, Z.H. Liu, B.Z. Li, B.E. Dale, Y.J. Yuan, Mass balance and transformation of corn stover by pretreatment with different dilute organic acids, Bioresour. Technol. 112 (2012) 319– 326. doi:10.1016/j.biortech.2012.02.134.
CrossRef - B. Yang, C.E. Wyman, Pretreatment: The key to unlocking low-cost cellulosic ethanol, Biofuels, Bioprod. Biorefining. 2 (2008) 26–40. doi:10.1002/bbb.49.
CrossRef - J. Placido, T. Imam, S. Capareda, Evaluation of ligninolytic enzymes, ultra-sonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash, Bioresour. Technol. 139 (2013) 203–208. doi:10.1016/j.biortech.2013.04.012.
CrossRef - J. Placido, S. Capareda, Analysis of alkali ultra-sonication pretreatment in bioethanol production from cotton gin trash using FT-IR spectroscopy, and principal component analysis, Bioresour. Bioprocess. 1 (2014) 1–9.
CrossRef - P. Alvira, E. Tom s-Pej, M. Ballesteros, M.J. Negro, Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review, Bioresour. Technol. 101 (2010) 4851–4861. doi:10.1016/j.biortech.2009.11.093.
CrossRef - C.N. Hamelinck, G. Van Hooijdonk, A.P.C. Faaij, Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term, Biomass and Bioenergy. 28 (2005) 384–410. doi:10.1016/j.biombioe.2004.09.002.
CrossRef - A.K. Chandel, O. V. Singh, M.L. Narasu, L.V. Rao, Bioconversion of Saccharum spontaneum (wild sugarcane) hemi cellulosic hydrolysate into ethanol by mono and co-cultures of Pichia stipitis NCIM3498 and thermotolerant Saccharomyces cerevisiae-VS 3, N. Biotechnol. 28 (2011) 593–599. doi:10.1016/j.nbt.2010.12.002.
CrossRef - B. Hahn-Hägerdal, K. Karhumaa, C. Fonseca, I. Spencer-Martins, M.F. GorwaGrauslund, towards industrial pentose-fermenting yeast strains, Appl. Microbiol. Biotechnol. 74 (2007) 937–953. Doi: 10.1007/s00253-006-0827-2.
CrossRef - A.K. Chandel, R.K. Kapoor, A. Singh, R.C. Kuhad, Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501, Bioresour. Technol. 98 (2007) 1947–1950. doi:10.1016/j.biortech.2006.07.047.
CrossRef - C. Pasha, M. Nagavalli, L. Venkateswar Rao, Lantana camara for fuel ethanol production using thermotolerant yeast, Lett. Appl. Microbiol. 44 (2007) 666–672. doi:10.1111/j.1472- 765X.2007.02116.x.
CrossRef - S.I. Mussatto, J.C. Santos, I.C. Roberto, Effect of pH and activated charcoal adsorption on hemi cellulosic hydrolysate detoxification for xylitol production, J. Chem. Technol. Biotechnol. 79 (2004) 590–596. doi:10.1002/jctb.1026.
CrossRef - P. Kumar, D.M. Barrett, M.J. Delwiche, P. Stroeve, Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production, Ind. Eng. Chem. Res. 48 (2009) 3713–3729. Doi: 10.1021/ie801542g.
CrossRef - C. Lu, H. Wang, Y. Luo, L. Guo, An efficient system for pre-delignification of gramineous biofuel feedstock in vitro: Application of a laccase from Pycnoporus sanguineus H275, Process Biochem. 45 (2010) 1141–1147. doi:10.1016/j.procbio.2010.04.010.
CrossRef - F. Nazarpour, D.K. Abdullah, N. Abdullah, R. Zamiri, Evaluation of biological pretreatment of rubber wood with white rot fungi for enzymatic hydrolysis, Materials (Basel). 6 (2013) 2059–2073. Doi: 10.3390/ma6052059.
CrossRef - B. Yang, Y. Lu, The promise of cellulosic ethanol production in China, J. Chem. Technol. Biotechnol. 82 (2007) 6–10.
CrossRef - M. Gutierrez-Correa, R.P. Tengerdy, Production of cellulase on sugar cane bagasse by fungal mixed culture solid substrate fermentation, Biotechnol. Lett. 19 (1997) 665–667. Doi: 10.1023/a: 1018342916095.
- M.A. Belewu, Conversion of masonia tree sawdust and cotton plant by product into feed by white rot fungus (), African J. Biotechnol. 5 (2006).
- W. Yang, F. Guo, Z. Wan, Yield, and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull, Saudi J. Biol. Sci. 20 (2013) 333–338.
CrossRef - G. Koutrotsios, K.C. Mountzouris, I. Chatzipavlidis, G.I. Zervakis, Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi- Assessment of their effect on the final product and spent substrate properties, Food Chem. 161 (2014) 127–135. doi:10.1016/j.foodchem.2014.03.121.
CrossRef - A.D. Moreno, D. Ibarra, P. Alvira, E. Tomas-Pejo, M. Ballesteros, A review of biological delignification and detoxification methods for lignocellulosic bioethanol production., Crit. Rev. Biotechnol. 8551 (2014) 1–13. doi:10.3109/07388551.2013.878896.
CrossRef - Nazhad MM, Ramos LP, Paszner L, Saddler JN. Structural constraints affecting the initial enzymatic hydrolysis of recycled paper. Enzyme Microb Technol 1995; 17(1):68–74.
CrossRef - Gonçalves DL, Matsushika A, de Sales BB, Goshima T, Bon EPS, Stambuk BU. Xylose, and xylose/glucose co-fermentation by recombinant Saccharomyces cerevisiae strains expressing individual hexose transporters. Enzyme Microb Technol 2014; 63:13–20.
CrossRef - Schmid RD. Pocket Atlas of biotechnology and genetic engineering. Ballan-Mire, French: Pocket Atlas; 2005.
- Dragone G, Silva DP, De Almeida E Silva JB. Factors influencing ethanol production rates at high-gravity brewing. LWT – Food Sci Technol 2004; 37(7):797–802.
CrossRef - Aldiguier AS, Alfenore S, Cameleyre X, Goma G, Uribelarrea JL, Guillouet SE, et al. Synergistic temperature and ethanol effect on Saccharomyces cerevisiae dynamic behaviour in ethanol biofuel production. Bioprocess Biosyst Eng 2004; 26(4):217–22.
CrossRef - Buzas Z, Dallmann K, Szajani B. Influenc of pH on the growth and ethanol production of free and immobilized Saccharomyces cerevisiae cells. Biotechnol Bioeng 1989; 34(6):882–4.
CrossRef - Rosa MF, Sa-Correia I. Intracellular acidification does not account for inhibition of Saccharomyces cerevisiae growth in the presence of ethanol. FEMS Microbiol Lett 1996; 135(2–3):271–4.
CrossRef







