Comparative Evaluation and Environmental Importance of Removal of Methyl Orange by Photocatalysis and MgO Nanoparticle
Corresponding author Email: shivaprasad.h61@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.17.1.15
Copy the following to cite this article:
Shivaprasad H, Nagarajappa D. P. Comparative Evaluation and Environmental Importance of Removal of Methyl Orange by Photocatalysis and MgO Nanoparticle. Curr World Environ 2022;17(1). DOI:http://dx.doi.org/10.12944/CWE.17.1.15
Copy the following to cite this URL:
Shivaprasad H, Nagarajappa D. P. Comparative Evaluation and Environmental Importance of Removal of Methyl Orange by Photocatalysis and MgO Nanoparticle. Curr World Environ 2022;17(1). Available From:
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 20-07-2021 |
|---|---|
| Accepted: | 04-03-2022 |
| Reviewed by: | 
 Kingshuk Dutta
Kingshuk Dutta
|
| Second Review by: |

 Animesh Debnath
Animesh Debnath
|
| Final Approval by: | Dr. M. Rafatullah |
Introduction
Removal of dyes from wastewaters discharged from industries like textile, leather tanning, pharmaceuticals, cosmetics, pigment etc is an important and challenging task. This is because the complex aromatic structure of dyes makes them more stable and more difficult to remove from wastewater. Several physico-chemical and biological methods viz coagulation and flocculation, biosorption, ultrafiltration, cation exchange membranes, electrochemical degradation, ozone treatment etc. have been used for removal of dyes from wastewaters.
Further the researchers opined that these methods suffer with major draw backs cannot be employed for efficient and satisfaction, cost effective treatment of coloured effluents. This necessitated the development of advanced technologies to address the safe disposal of dye effluents.
The researchers opined that the photocatalytic processes and nanomaterials can prove economical ecological friendly in treating waste streams. Nanotechnology has been considered effective in solving wastewater treatment and disposal problems.
Amin et al; (2014) reviewed the application of nano materials in removing pollutants from water/ wastewaters. In particular, removal of dyes from wastewaters by nanomaterials is reviewed.
Dharmendra et al (2008) presented a paper on “Application of nanoparticles in wastewater treatment” researchers across the globe published papers on feasibility of using nanomaterials. The results indicated the higher degradation of the colour by zinc oxide they inferred that the main reason for this is zinc oxide absorbs large fraction of the solar spectrum compared to titanium dioxide. Amongst the various advanced techniques employed for wastewaters treatment, photocatalysis is regarded as most viable one.Catalyst may accelerate the photo reaction by interaction with the substrate in its ground or exited state depending upon the mechanism of photoreaction. Even though the research addressing photocatalytic degradation and nanotechnology is in progress across the globe, the technologies are wastewater specific and are functions of many experimental variables. This paper focus on the use of MgO nanoparticle in the photocatalytic decolourisation of methyl orange and also simply as a nanoparticle.
Materials and Methods
Synthesis of Magnesium Oxide (MgO) Nanoparticles
The synthesis of nanoparticle is carried out as per the procedure given by Daniel and Shobha (2015). These nanoparticles are synthesised by a chemical method. 6g Mgcl2 and 2g Surfactant Sds (Sodium dodecyl Sulphate) are mixed in 100 ml of double distilled water. This mixture is added into 0.4 N NaOH solution and constantly stirred on a magnetic stirrer for about 2h by maintaining the pH 11. Further precipitate of Mg (OH)2, white in colour is rinsed thoroughly with carbonated water. The precipitate is then desicated at 120oC for 2h then calcinated for 5 hours in a muffle furnance at 800oC. The contents are then allowed to cool to attain room temperature and to obtain granular nano MgO.
Photocatalytic Experiment
A set of photocatalytic degradation experiments are conducted in Borocil Silicate gas cylinder (Photo reactor) under UV light irradiation. The light source is mercury lamp of 125 watts. The experiments are performed at ambient temperature the experiments are conducted to evaluate optimum photocatalytic condition such as pH, Irradiation time, Initial concentration of time but keeping constant photo catalytic dosage of 0.3 g/L. The reactor is placed on a magnetic stirrer and contents are stirred during irradiation to keep the suspension homogenous. After specific predecided times of irradiation suitable aliquiots of samples are withdrawn and analyzed after centrifugation using UV Spectrophotometer.
Batch Studies
The batch studies are conducted in an open atmosphere at room temperature. 100 ml of coloured sample of predecided colour concentration (10,40,70 and 90 mg/L) is taken in a clean dry conical flask. By adding acid or alkali the pH of the sample is adjusted. To discontented in a conical flask adsorbent (MgO) dosage at the rate of 0.3 g/L added. The contents of the flask are stirred by using glass rods followed by shaking at 140 rpm in a shaker for selected stirring time (varied from 15 to 60 min). Further the treated dye solutions are centrifuged for 5 minutes at 3000 rpm to exclude the adsorbent particle if any. The clear solution is piped out and the rate of decolorization is measured using UV Spectrophotometer employing an optimum wavelength of 465nm. By using a calibration curve prepared for methyl orange, percentage of decolorization is determined.
Adjustment of pH
pH of aqueous coloured samples (3 to 9) is adjusted by using H2SO4 or NaOH as applicable.
Preparation of Aqueous Coloured Samples
Commercially available methyl orange dye is procured from colour text by dissolving 1g of dye in 1litre of double mineral water the stock solutions of concentration 1000 mg/L is prepared. Further by diluting calculating the quantity of assortment using double distilled water colour concentration of samples adopted for present work are prepared.
Environmental Importance of Mgo
Magnesium oxide nanoparticles are also known to have a number of benefits, including low phytotoxicity, thermal stability, non-genotoxicity, and non-biotoxicity to people, making them ideal for a variety of applications. MgO nanoparticles play an important role in environmental remediation by treating waste water, industrial and domestic waste, soil sediments, and air pollution. Magnesium oxide nanoparticles have a variety of properties, including anti-biofilm activity, self-cleaning activity, and the ability to remove phosphorus from wastewater, which is a reason for plant growth inhibition.
Results and Discussions
The findings of Batch studies under varied experimental condition are showed in fig 1,2,3 and 4. Based on the analysis of experimental findings the inferences are drawn and documented on below.
 |
Figure 1: Effect of Stirring Time on Removal Efficiency of Colour (Batch Studies; Co=10 mg/L) Click here to view Figure |
 |
Figure 2: Effect of Stirring Time on Removal Efficiency of Colour (Batch Studies; Co=40 mg/L) Click here to view Figure |
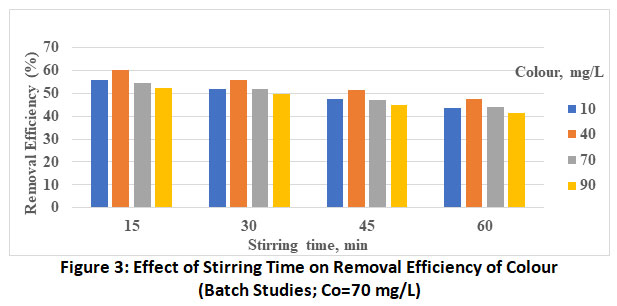 |
Figure 3: Effect of Stirring Time on Removal Efficiency of Colour (Batch Studies; Co=70 mg/L) Click here to view Figure |
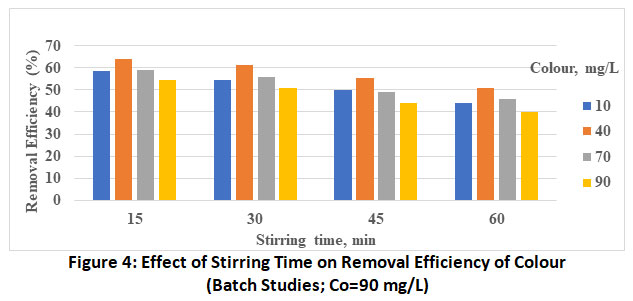 |
Figure 4: Effect of Stirring Time on Removal Efficiency of Colour (Batch Studies; Co=90 mg/L) Click here to view Figure |
The Effect of pH on Adsorption
In the present studies the optimum removal is recorded at pH 5 (acidic medium) under all varied condition of experimentation. It is opined that at the lower pH adsorbent surface become protonated.
Effect of Initial Dye Concentration
The effect of initial dye concentration in the range of the 10 to 90 mg/L is investigated presented in bar charts (figure 1 to 4) decreasing percentage removal with increasing the initial concentration as been adsorbed. These values at optimum pH and stirring of 5 and 60 minutes respectively are found to vary from 63.9% (Co=10 mg/L) to 50.7% (Co=90 mg/L).
Effect of Stirring Time
The plots revealed the increase in the rate of adsorbent with increase in stirring time from 15 to 60 min. This is attributed to more energy provided by stirring which might have brought the colour molecules from the bulk of the solution to active site of the adsorbent. Thus, the maximum removal efficiency of methyl orange by MgO nanoparticle is found to be 63.9%, the optimum experimental conditions being, Co=10 mg/L, stirring time=60 min and pH-5.
Photocatalytic Studies
The outcome of nano photocatalytic studies carried out to access the nano photocatalytic degradation of methyl orange using MgO nano particle under varied experimental condition are presented in figure 5. An attempt as been made to analyse the effect of irradiation time, pH and initial dye concentration. The discussions are documented as below.
Effect of pH
pH is the operatingparameters which affect the adsorption of pollutants by the photocatalytic on their surfaces. pH affects the charge on the catalyst particle and the position of conductance and valence bonds (Abdul Raheem et al;2012)
Further the literature review revealed that the interpretation of efficiency of photo degradation process with respect to pH is very complicated process, because of multiple roles of pH. Possible reaction mechanisms involved in photocatalytic process of pollutant covering oxidation and reduction, point of zero charge of the photo catalyst, ionic specifications etc. are discussed or published by various researchers.
Photocatalytic effect of pH as degradation of colours (Azo dyes, anionic dyes, organic dyes, reactive dyes etc) has beenstudied by investigators. The studies revealed that effect of pH on removal efficiency of colours is a function of photo catalyst and colour in particular.
Byrappa et al; (2006) based on their studies on the photocatalytic degradation of Rhodamine B dye using ZnO nanoparticle reported high degradation efficiency at neutral pH as well as good degradation at extreme acidic and alkalic pH. Also reported higher degradation efficiency of reactive violet 5 at both solution pH of less than 5 and greater than 9. They inferred that in alkaline solution is likely to be adsorbed on negatively charged nano particle surface and in acidic conditions on positively charged nano particle surfaces.
Further the studies also observed no considerable change in process efficiency over a wide range of pH 4 to 8 in photocatalytic degradation of anionic dye by TiO2 nanocatalyst. However, the presence studies shown optimum removal of methyl orange at acidic pH of 5 under all varied conditions of experimentations.
Effect of Irradiation Time
Figure 5 shows the relationship between the photodegradation efficiency of methyl orange and the irradiation time. It can be clearly seen that the discoloration and deterioration rate increase as the irradiation time increases, and these trends have been reported by many investigators worldwide. This results in higher efficiency with higher irradiation time. In the present work maximum photocatalytic degradation of methyl orange by MgO nano particle is observed at irradiation time of 50 minutes and removal efficiency at irradiation time of 10 minutes.
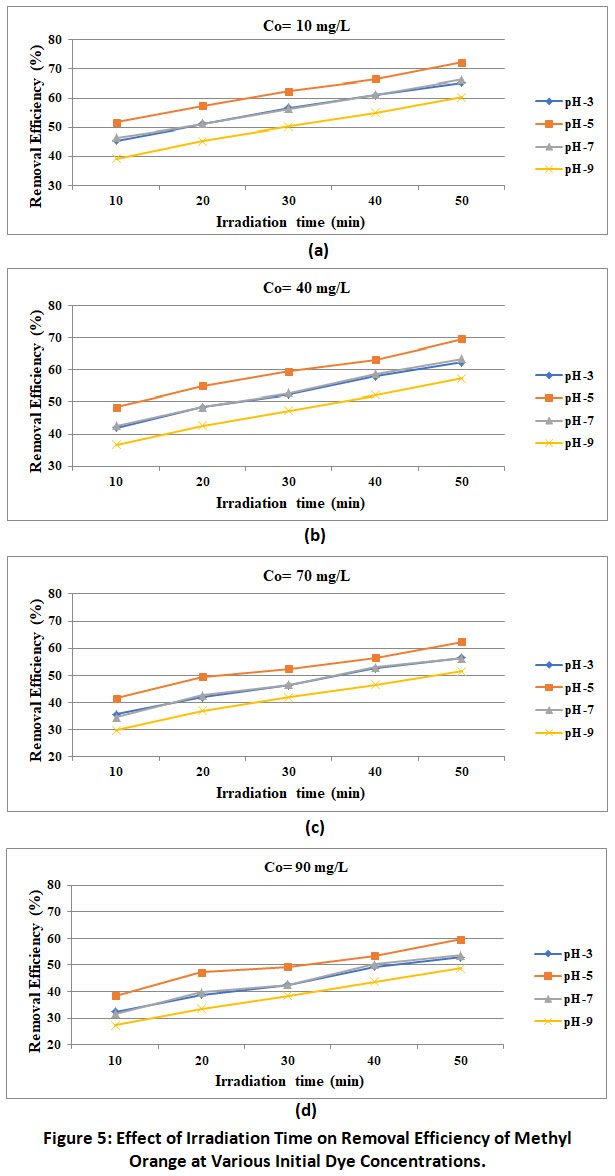 |
Figure 5: Effect of Irradiation Time on Removal Efficiency of Methyl Orange at Various Initial Dye Concentrations. Click here to view Figure |
Effect of Initial dye Concentration
Inpresent workinfluence of initial dye concentration varying from 10 to 90 mg/L at different pH of the solution and irradiation time are tried. The results of experimentation as presented in figure 5 revealed the inverse relationship between the initial concentration and removal efficiency maximum and minimum removal efficiencies of 72.3% and 59.3% respectively are recorded under the optimum condition of experimentations corresponding into the initial dye concentration of 10 mg/L and 90 mg/L respectively. Same trends are reported by Saktivel et al;(2003), Sampa and Binyl(2004), Abdul Raheem et al;(2012).
Adsorption Isotherms Studies
Amongst the various adsorption models, Langmuir and Freundlich models found to have wider applications.
The concept, theory and applications are in-depth discussed and published by researchers and could be found in literature. But here in this paper an attempt has been made only tohighlight equations of these isotherms, determination of constants and validity.
The Langmuir model can be represented by the equation;
Ce/qe =1/qmb+Ce/qmax
The linear plots of Ce/qe V/S Ce suggest the applicability of Langmuir isotherm. The qmax is determined by slope and b will be the intercept of the line.
To predict whether the Langmuir type of adsorption is favorable or unfavorable, a dimensionless equilibrium parameter, denoted by RL is also known as the separation factor. The following equation can be used for calculating RL.
RL=1/1+bCo
The value of RL between 0 and 1 indicate the favorable condition.
Freundlich isotherm is an exponential equation and can be
qe = KfCe 1/n
The equation can be rearranged as below;
logqe = logKf + 1/n logCe
where qe and Ce are same as defined for Langmuir isotherm
A plot of logqe V/SlogCe yields a straight line, wherein slope represents 1/n and intercept represents logKf. Values of 1/n<1 indicate the favourable. Further R2 values close to 1.0 validate the above two isotherms. The data obtained by the experimentation in the present study is used to plot Langmuir and Freundlich isotherms under shown in figure 6 and 7.
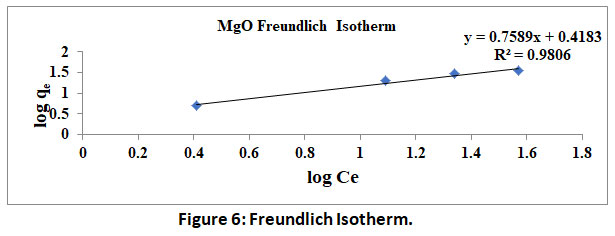 |
Figure 6: Freundlich Isotherm. Click here to view Figure |
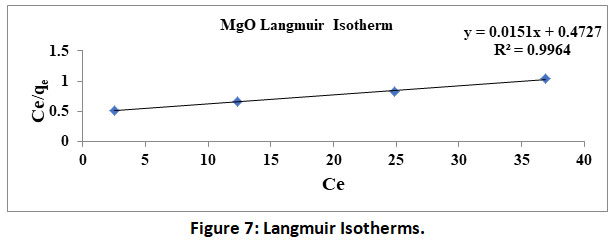 |
Figure 7: Langmuir Isotherms. Click here to view Figure |
Further the isotherms constants calculated from the table 1. RL and 1/nvalues are found to be less than 1 and further correlation coefficient R2 for both the isotherm are found to be very high and are comparable within statistical limitations. Thus, the constants/parameters and coefficients calculated/observed confirm the fitment of experimental data into both the isotherms.
Table 1: Isotherms Constants.
|
Isotherms |
Parameters |
|||||
|
qmax(mg/g) |
b (l/mg) |
RL |
Kf |
1/n |
R2 |
|
|
Langmuir |
153.84 |
0.059 |
0.628 |
- |
- |
0.9969 |
|
Freundlich |
- |
- |
- |
9.86 |
0.7404 |
0.9905 |
Conclusions
The outcome of experimentation carried out to investigate the adsorption potential of methyl orange on to MgO by batch studies and by nano photocatalysis are presented in this paper. Attempt also been made to validate the experimental data for Langmuir and Freundlich isotherms. The removal efficiency is found to be directly proportional to irradiation time/stirring time and inversely proportional to initial concentration of dye solutions. Maximum removal efficiency has been adsorbed at acidic media. At optimum conditions of experimental variables tried viz Co=10 mg/L, pH-5, irradiation time/stirring time= 50 min, adsorption dosage= 0.3 g/L, maximum removal efficiencies of 63.9% and 72.3% respectively are recorded with adsorption of nanoparticles and nano photocatalysis. It is concluded that the colour tried can be better removed from aqueous suspension by nano photocatalysis. However, it is suggested to work out cost economics of these two systems before deciding their applications. Further it is also suggested to evaluate the systems for higher adsorbent dosages.
Acknowledgements
Acknowledgement to the Principal, Head of the department in Civil Engineering, Research supervisor and lab instructors in University B D T College of Engineering, Davanagere which has provided help during the research work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
There is no conflict of interest.
References
- Amin, M. T., Alazba, A. A. and Manzoor, U., 2014. “A review of removal of pollutants from water/wastewater using different types of nanomaterials”. Adv. Mater. Sci. Eng., 1(5):125-146.
CrossRef - Dhermendra K. Tiwari, J. Behari and Prasenjit Sen “Application of Nanoparticles in Waste Water Treatment” World Applied Sciences Journal 3(3):417-433, 2008 ISSN 1818-4952DOI:10.15680/IJIRSET.2016.0508217.
- Sara Teixeira, Robert Gurke, Hagen Eckert, Klaus Kuhn, Joachim Fauler, GianaurelioCuniberti, “Photocatalytic degradation of pharmaceuticals present in conventional treated wastewater by nanoparticle suspensions” Journal of Environmental Chemical Engineering 4 (2016) 287–292.
CrossRef - G. Suvarna Lakshmi, M.V.V. Chandana Lakshmi “Removal of Organic Pollutants from the Pharmaceutical Effluent by TiO2 Based Photocatalysis” International Journal of Innovative Research in Science, Engineering and Technology. 5, Issue 8, August 2016 SSN(Online): 2319-8753 ISSN (Print): 2347-6710DOI:10.15680/IJIRSET.2016.0508217.
- S. Ghanbarnezhad, L Sharifi, S H Mirhosseini, V Khani and S H Zare “The Influence of Bentonite on the Removal of Methyl Violet Dye” Vol 70 (2014), pp47-54 International Journal of Advanced Science and Technology ISSN:2005-4238 IJAST Copyright @ 2014 SERSC DOI: 10.14257/ijast.2014.70.05.
CrossRef - AbdulraheemGiwa, Peter Obinna Nkeonye, Kasali Ademola Bello, Kasali Ademola Kolawole “Photocatalytic Decolourization and Degradation of C. I. Basic Blue 41 Using TiO2 Nanoparticles” Journal of Environmental Protection, 2012, 3, 1063-1069 http://dx.doi.org/10.4236/jep.2012.39124 Published Online September 2012 (http://www.SciRP.org/journal/jep)DOI: 10.4236/jep.2012.39124.
CrossRef - B.L.Dinesha, H. Sharanagouda, N. Udaykumar, C.T. Ramachandra and Anil B. Dandekar “ Removal of Pollutants from Water/Waste Water Using Nano-Adsorbents: A Potential Pollution Mitigation”International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 10 (2017) pp. 4868-4872 Journal homepage: http://www.ijcmas.com DOI: 10.20546/ijcmas.2017.610.455.
CrossRef - C. Namasivayam, D Kavitha “Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste” 47-58, Dyes and pigments 54 (2002) Elsevier Science Ltd. DOI: https://doi.org/10.1016/S0143-7208(02)00025-6.
CrossRef - Kansal SK, Singh M, Sud D. (2007) Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. Journal of Hazardous Materials 141(3), 581–90.
CrossRef - Konstantinou IK, Albanis TA. (2004) TiO2 -assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations-A review. Applied Catalysis B: Environmental 49(1), 1-14.
CrossRef - Sampa chakrabarti, Binay K. Dutta, “Photocatalytic degradation of model textile dyes in wastewater using TiO2 as semiconductor catalyst”, Elsevier, vol. 5, no.13, pp. 0304-3894, 2004.DOI:10.15680/IJIRSET.2016.0508217.
- Akpan UG, Hameed BH. (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2 -based photocatalysts: A review. Journal of Hazardous Materials 170(2-3), 520-9.
CrossRef - Albanis TA. (2004) TiO2 -assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations-A review. Applied Catalysis B: Environmental 49(1), 1- 14.
CrossRef - Byrappa et al., “Synthesis, characterization and adsorption behavior of MgO nanoparticles on Rhodamine B dye” Journal of chemical and pharmaceutical research (2014)7(8),713-723DOI:10.1016/S0143-7208(02)00025-6.
CrossRef - Daniel, Shobha V S (2015) “Synthesis, characterization and adsoption behavoiur of MgO nanoparticles on Rhodamine B dye” Journal of chemical and pharmaceutical research 7(8),713-723
- Sakthivel S, Neppolian B, Arabindoo B, Palanichamy M, Murugesan V. (2000) TiO2 catalysed photodegradation of leather dye. Journal of Scientific & Industrial Research 59(7), 556-62.







