Biosynthesis of Silver Nanoparticles and its Utilization in Dye Decomposition for a Clean Environment: A Step Towards Sustainable Development
1
Department of Physics,
Balarampur College, P.O- Rangadih,
Dist-Purulia,
West Bengal
India
Corresponding author Email: tatanghosh83@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.17.2.6
Copy the following to cite this article:
Ghosh T. Biosynthesis of Silver Nanoparticles and its Utilization in Dye Decomposition for a Clean Environment: A Step Towards Sustainable Development. Curr World Environ 2022;17(2). DOI:http://dx.doi.org/10.12944/CWE.17.2.6
Copy the following to cite this URL:
Ghosh T. Biosynthesis of Silver Nanoparticles and its Utilization in Dye Decomposition for a Clean Environment: A Step Towards Sustainable Development. Curr World Environ 2022;17(2).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-06-13 |
|---|---|
| Accepted: | 2022-08-25 |
| Reviewed by: | 
 V. Mohan Raj
V. Mohan Raj
|
| Second Review by: |

 Animesh Debnath
Animesh Debnath
|
| Final Approval by: | Dr Gopal Krishan |
Introduction
Synthesis of material in the nanometer scale is the remarkable discovery in the twentieth century. The word “Nano” derives from the ancient Greek word “nanos” which means “dwaft”. Nano refers to one billionth. Nanometer (nm) can be felt by saying that a human hair is about one million nm wide. A material is called nanomaterial when at least one of its sides belongs to 1-100 nm. Nanomaterials show significantly different physical and chemical properties in comparison to their bulk form. For example, the opaque material turns transparent (e.g. copper), insoluble becomes soluble and chemically inert substance acts as an efficient catalyst (e.g. gold) when they are in nano form 1. Metal nonoparticles (NPs) offer uniqueness in optical properties as because of surface plasmon resonance absorption. The thrilling colour of the nanomaterial appears not only due to its size or shape but also due to the dielectric medium surrounding them. The gold is yellow when in bulk form but the colour of gold colloid changes from ruby red to bluish as soon as the size of the particle varies from 20 nm to 200 nm, respectively 2.
Noble metal NPs such as platinum, palladium, gold and silver (Ag) receive much attention because of their promising applications in advanced technology 3. Among these metal nanoparticles, Ag has been chosen in promising biomedical application due to its high stability, cost effectiveness and high efficiency in contrast to the rest 4. Apart from the increasing industrial use of Ag NPs, they are equally used in different medical applications including imaging, hyperthermia of tumours, drug delivery and even as an anti-carcinogen 5.
The increasing global population with rapidly mutable microbial pathogens necessities the inevitable discovery of novel drugs to combat against human diseases. Also, the indiscriminate production and unscientific use of drugs without proper medical advice results in emergence of multiple antibiotic resistant strains (MARS) producing the more complicated issues of health hazard 6. So, it is an alarming state for the scientists to develop novel drug for fighting against MARS. In this context, nanomaterials were paid much attention by the researchers due to their strong antimicrobial action, smallness in size resulting higher surface area, and strong binding affinity with microbes 7. From the long past silver was utilized as a competent antimicrobial material used in medical purposes 8. Ag NP sets its goal as it exhibits significant antimicrobial effect against a wide variety of microorganisms 9.
For synthesizing Ag NPs a lot of methods are available few of which are laser ablation, pyrolysis, chemical method, microwave assisted method, hydrothermal method etc. 10. Each of these methods suffers one or more drawbacks which include cost effectiveness, energy consumption, utilization of toxic chemicals such as capping and stabilizing agents, organic solvent and hence none is sustainable. To overcome this problem a new synthesis method called green synthesis method following the green chemistry to synthesize Ag NP is a sustainable way for better future 11,12. Here in this method no toxic chemical was used and hence it is environment friendly as well as cost effective. Green synthesis method is either plant mediated or microbe mediated 13. Due to the cultural hazards of microorganisms, researchers usually prefer to use different parts of various plants as green materials for synthesizing nanomaterials. Ag NPs have already been synthesized by us using both the aqueous extracts of Neem and Guava leaf separately 12-14. In this study we report the formation of Neem leaf extract mediated Ag NPs prepared at normal temperature using the aqueous solution of only one chemical which is silver nitrate. The formation of Ag NP was confirmed by optical absorption spectra whereas the size of the particle was estimated by capturing the image of the particles by a transmission electron microscope. The results of all the experiments confirm that the Ag NPs were synthesized successfully in this sustainable green synthesis method.
During the last few decades the water is getting polluted by the organic dyes discharged from various industries leading to an untoward effect in environment as well as human health. Although a large number of methods like filtration, coagulation, ozonation, adsorption, anodic oxidation, reverse osmosis, etc. are being available for the removal of the toxic dyes, they all are not cost-effective 15. Recently, the removal of methyl red dyes from aqueous solution using a novel hybrid absorbent PANI@Fe-Mn-Zr 16 and MgFe2O4/polyaniline nanocomposite 17 has been reported. A quick and enhance separation of the toxic dye Eosin Yellow from its aqueous solution has been achieved by coagulation method using ferric chloride as a coagulant 18. So there is a growing demand towards a fast, improved, effective and low cost method for the removal of the hazardous dyes. Recently, green synthesized metal NPs has been used for the decomposition of different dyes such as methylene blue, rhodamine B, methyl red, 4-nitrophenol, congo red, and methyl orange 19. However, the work claims its novelty as a significant percentage (~88%) of degradation of methylene blue (MB) dye under sunlight illumination has been achieved by using biosynthesized Ag NPs prepared by following the green chemistry.
Synthesis and characterization
The schematic diagram for synthesis of biogenic Ag NPs is shown in figure 1. Fresh Neem leaves were collected from the local area and were washed properly. The leaves were sun dried and then crushed into powder. Approximately 5 gm of leaf dust was added in 0.1 liter distilled water and was kept at 45º C inside in a hot air oven till the water turns into light yellow. The coloured extract was then filtered properly and preserved in a refrigerator. The preparation of the sample is same as described in our previous work [12,14]. Here 2 ml leaf extract was added in 40 ml aqueous solution of silver nitrate (5 mM) and the mixture was stirred continuously. After a while, the colour of the colourless mixture gradually turns into light yellow and then blackish yellow confirming the formation Ag NPs.
.jpg) | Figure 1: Schematic diagram showing the synthesis of biogenic Ag NPs
|
Optical absorption(OA) spectroscopy measurements were performed using UV-Vis spectrophotometer (JASCO, V-630). The crystal structure of the sample was studied through X-ray diffraction (XRD) techniques. Here PROTO AXRD diffractometer was used. It was functioning at 0.6 kW with operating X-ray wavelength 1.540 Å (Cu K? radiation). For recording the XRD data, film was made by putting a couple of drops of the liquid sample on a cleaned glass substrate. The images of the particles were obtained by using a transmission electron microscope (JEOL, JEM 1400 plus) 12,14.
Study of dye degradation activity
The dye degradation mechanism of these Ag NPs was performed on methylene blue dye under sunlight illumination. The dye solution was made by mixing 1 mg methylene blue in 0.2 liter distilled water. Then 5 mg of Ag NPs was added into the solution and mixture was stirred for 30 minutes in a dark chamber. The solution was then kept under sunlight illumination with continuous stirring. At a particular time interval some amount (~2 ml) of coloured water was isolated from the mixture and OA study was performed. The recording of data of this degradation was done by JASCO, V-630 UV-Visible spectrophotometer 20.
Experimental Result
Optical absorption (OA) spectroscopy
At the time of preparation of sample, a gradual colour change of the aqueous sample was noticed as already discussed. Such change in colour confirms the formation Ag NP with different sizes. The formation of Ag NP can be confirmed by measuring the OA spectra of the coloured aqueous sample. It is the most essential and elementary techniques. Figure 2 shows the OA spectrum of the synthesized sample after 2 hours of adding Neem leaf extract in the silver nitrate solution. The spectrum contains a broad absorption peak centered at ~420 nm which confirms the formation of NPs of Ag. This absorption also called surface plasmon resonance (SPR) arises when the unbound electron on the metal surface oscillates harmonically when excited by electromagnetic radiation 21.
TEM Studies
The direct measurement of size of the NP is done using the image as obtained from transmission electron microscope. Figure 3 shows the corresponding image where the particles are found to be nearly spherical. It is estimated that the average size of the synthesized sample is 30 nm.
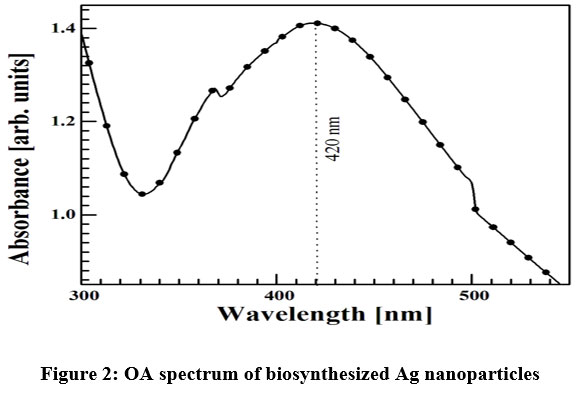 | Figure 2: OA spectrum of biosynthesized Ag nanoparticles
|
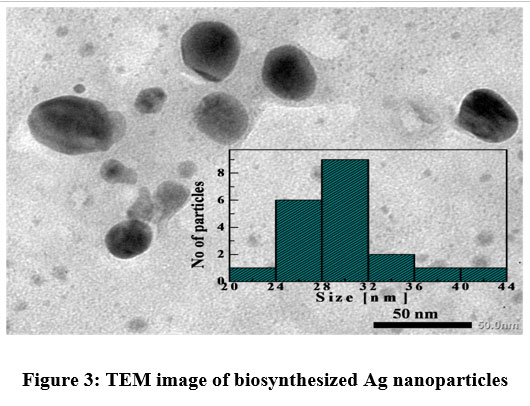 | Figure 3: TEM image of biosynthesized Ag nanoparticles
|
XRD studies
Material identification and crystallinity of the material was analyzed by X-ray diffraction (XRD) spectrum. Figure 4 shows the XRD spectrum of the sample. The spectrum consists of four prominent diffraction peaks at ~ 38º, 44º, 54º and 78º corresponding to (111), (200), (220) and (311) planes of Ag 22. The diffraction peak marked as (*) arises due to presence of biomolecules 23 within the sample. This result confirms that the synthesized sample is face-centred cubic and well crystalline structure. From XRD data mean grain size of particle is estimated as ~20 nm 23.
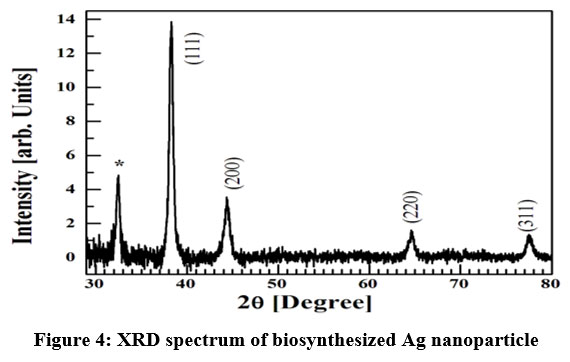 | Figure 4: XRD spectrum of biosynthesized Ag nanoparticle
|
Energy dispersive X-ray spectroscopy (EDS) Study
Figure 5 shows the EDS spectrum the synthesized sample. The spectrum contains a strong peak of Ag confirming the chemical composition of the synthesized sample. The other small peaks of C, O and Cl in the EDS spectrum may due to the X-ray emission from biocompounds responsible for stabilization of the nanoparticle present in the sample 24. The obtained result is in line because Ag NPs usually show a characteristic OA peak at around 3000 eV because of SPR 25.
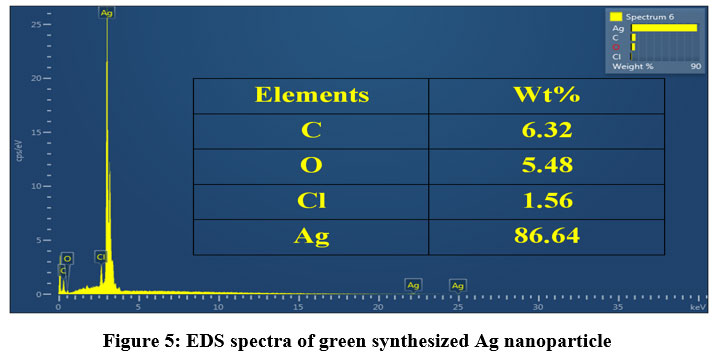 | Figure 5: EDS spectra of green synthesized Ag nanoparticle
|
Evaluation of Dye degradation
The study of photocatalytic activity was performed on the decomposition of MB in sunlight illumination. Figure 6 shows the degradation of the dye at different time. Each spectrum consists of two OA peaks centered at ~663 nm and ~610 nm which ascribes the transitions of and, respectively 26. The intensity of the absorption peaks steadily decreases with time which means that the biogenic Ag NPs decompose the dye and degrade it.
The percentage of degradation of the dye is estimated by using the equation as follows 27
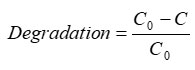
Where C0 and C are respectively the concentration of the dye at time and at any time t.
The concentration of MB dye is proportional to the absorbance. Figure 7 shows the variation of percentage of dye degradation with time. It is observed that the dye degrades up to 88 % in four hours which infers the excellent performance of biogenic Ag NPs as a photocatalyst. Table 1 shows a comparison study of previously reported result with the present result on green synthesized Ag NPs mediated MB dye degradation in presence of sunlight. From the table it can be concluded that our green synthesized Ag NPs exhibited better photocatalytic effect in comparison to others.
Table 1: Comparison of Ag NPs mediated MB dye degradation in sunlight illumination of present study with some previously reported result
Extract used for Ag NPs synthesis | Concentration of MB dye (mg/l) | Concentration of Ag NPs (mg/l) | Time (hour) | Degradation (%) | References |
Casuarina equisetifolia | 1 | 100 | 5 | 35-40 | [28] |
Morinda tinctoria | 10 | 100 | 72 | 95.3 | [29] |
Pomegranate peel | 10 | 100 | 48-72 | 89 | [30] |
Durio Zibethinus seed waste | 10 | 100 | 3 | 73.49 | [31] |
Natural honey | 10 | 100 | 72 | 92 | [32] |
Prosopis farcta fruit | 10 | 500 | 4 | 86.38 | [33] |
Neem leaf | 5 | 25 | 4 | 88 | This study |
By absorbing the solar photons, excitation of surface plasmon electrons of Ag nanoparticles occurs. These excited electrons get absorbed by the dissolved oxygen molecules in the medium and transform them into superoxide oxygen radicals (•O2-) which is a very strong oxidizing agent capable to decompose the MB dye and degrade it [34]. This explains how Ag NPs degrade MB dye under solar light irradiation.
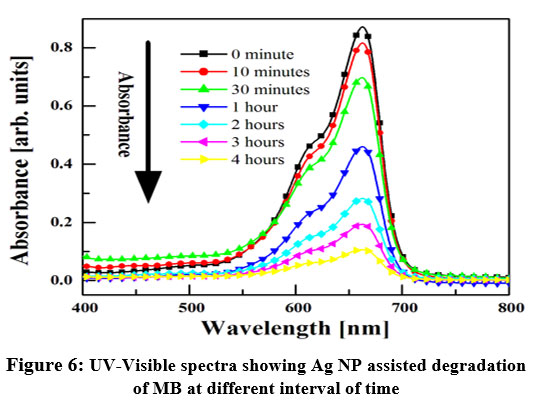 | Figure 6: UV-Visible spectra showing Ag NP assisted degradation of MB at different interval of time
|
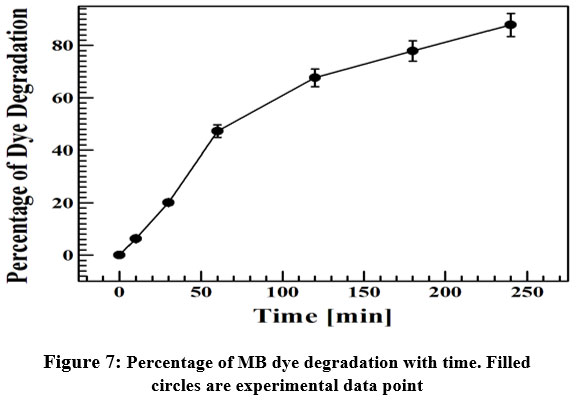 | Figure 7: Percentage of MB dye degradation with time. Filled circles are experimental data point
|
Conclusion
Here we have successfully synthesized silver nanoparticle at room temperature by using aqueous extract of Neem leaf and silver nitrate solution by green synthesis approach. No extra chemical other than silver nitrate has been used here and hence it is eco-friendly and sustainable. Here the different biocompounds present in extract of Neem leafserves reducing as well as capping agent. The formations of Ag nanoparticle was primarily confirmed through the colour change of the aqueous solution of silver nitrate and experimentally confirmed by optical absorption spectrum showing a broad absorption peaks at ~420 nm. The size of the particle was estimated by the image obtained by transmission electron microscope and found to be in the nanometer (10-9 m) scale. All the experimental findings are in line with the others’ observational results and our previous work. The synthesized Ag nanoparticles are well crystalline and pure crystal of Ag as no other peak apart from the characteristic peaks is observed in XRD spectra. Finally it is to be concluded that, the biosynthesized Ag nanoparticles can be used in waste water treatment, textile industry or elsewhere as a photocatalytic agent for dye degradation in order to reduce toxic effects of synthetic dyes that pollutes the environment.
Acknowledgement
The author would like to acknowledge the Department of Physics, Sidho-Kanho-Birsha University, Purulia, West Bengal and University Science Instrumentation Centre (USIC) of The University of Burdwan for extending experimental facilities. The author would also like to acknowledge Prof. Probodh K. Kuiri, Professor, Department of Physics, Sidho-Kanho-Birsha University and Prof. Atis C. Mandal, Professor, Department of Physics, The University of Burdwan for their motivations and inspirations.
Conflict of Interest
The author declares no conflict of interest.
Funding Sources
The author received no financial support for the research, authorship, and/or publication of this article.
References
- Poole Jr. C. P, Owens F. J. Introduction to Nanotechnology. New Jersey: Wiley Interscience, 2003.
- Eustis S, Sayed M. A. E. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006; 35(3): 209-217.
CrossRef - Prabhu S, Poulose E. K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano Lett. 2012; 2: 32.
CrossRef - Lara H. H, Garza-Trevino E. N, Ixtepan-Turrent L, Singh D. K.Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnology. 2011; 9: 30.
CrossRef - Daniel M. C, Astruc D. Gold Nanoparticles:? Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004; 104: 293-346.
CrossRef - Srinivasan A, Lopez-Ribot J. L, Ramasubramanian A. K. Overcoming antifungal resistance. Drug Discov Today Technol. 2014; 11: 65-71.
CrossRef - Morones J. R, Elechiguerra J. L, Camacho A, Holt K, Kouri J. B, Ramirez J. T, Yacaman M. J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005; 16(10): 2346-2353.
CrossRef - Rauwel P, Küünal S, Ferdov S, Rauwel E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015; 2015: 682749.
CrossRef - Yin I. X, Zhang J, Zhao I. S, Mei M. L, Li Q, Chu C. H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020; 15: 2555–2562.
CrossRef - Patra S, Madhuri R. Green Synthesis of Noble Metal Nanoparticles: A Step Forward to Economical and Sustainable Development: Synthesis, Characterization and Their Applications, Suvardhan Kanchi and Shakeel Ahmed (eds). Wily Online Library; Green Metal Nanoparticles. 2018:555–602.
CrossRef - Verma R, Pathak S, Srivastava A. K, Prawer S, Hanic S. T. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J Alloys Compd. 2021; 876: 160175.
CrossRef - Ghosh T, Chattopadhyay A, Mandal A. C, Pramanik S, Kuiri P. K. Optical, structural, and antibacterial properties of biosynthesized Ag nanoparticles at room temperature using Azadirachta indica leaf extract. Chinese J. Phys. 2020; 68: 835-848.
CrossRef - Debnath B, Majumdar M, Bhowmik M, Bhowmik K. L, Debnath A, Roy D. N. The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology. J. Environ. Manage. 2020; 261:110235.
CrossRef - Ghosh T, Chattopadhyay A, Mandal A. C, Pramanik S, Mukherjee S, Kuiri P. K. Spectroscopic, microscopic and antibacterial studies of green synthesized Ag nanoparticles at room temperature using Psidium guajava leaf extract. Korean J Chem. Eng. 2021; 38(12): 2549-2559.
CrossRef - Dutta S, Gupta B, Srivastava S. K, Gupta A. K. Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Materials Advances. 2021; 2 (14): 4497-4531.
CrossRef - Saha B, Debnath A, Saha B. Fabrication of PANI@Fe-Mn-Zr hybrid material and assessments in sono-assisted adsorption of methyl red dye: Uptake performance and response surface optimization. J. Indian Chem. Soc. 2022; 99(9):100635.
CrossRef - Das P, Debnath A. Fabrication of MgFe2O4/polyaniline nanocomposite for amputation of methyl red dye from water: Isotherm modeling, kinetic and cost analysis. J. Dispers. Sci. Technol. 2022; DOI: 10.1080/01932691.2022.2110110.
CrossRef - Paul S. R, Singh N. H, Debnath A. Quick and enhanced separation of Eosin Yellow dye from aqueous solution by FeCl3 interaction: thermodynamic study and treatment cost analysis. International Journal of Environmental Analytical Chemistry. 2022. DOI: 10.1080/03067319.2022.2076218
CrossRef - Muthu K, Rajeswari S, Akilandaeaswari B, Nagasundari S. M, Rangasamy R. Synthesis, characterisation and photocatalytic activity of silver nanoparticles stabilised by Punica granatum seeds extract. Materials Technology. 2021; 36(11):684-693.
CrossRef - Ghosh T, Chattopadhyay A, Pramanik S, Das A, Mukherjee S, Das S, Mandal A. C, Dhak D, Kuiri P. K. Role of Ag Nanoparticles on Photoluminescence Emissions, Antibacterial Activities, and Photocatalytic Effects in ZnO-Ag Nanocomposites Synthesized via Low Temperature Green Synthesis Method Using Azadirachta Indica Leaf Extract. Materials Technolgy. 2022. DOI: 10.1080/10667857.2022.2029290.
CrossRef - Siddhant J, Mehata M. S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017; 7: 15867.
CrossRef - Goudarzi M, Mir N, Kamazani M. M, Bagheri S, Niasari M. S. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci. Rep. 2016; 6: 32539.
CrossRef - Verma A, Mehata M. S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016; 9(1):109-115.
CrossRef - Syed B, Prasad M. N. N, Dhananjaya B. L., Kumar K. M, Yallappa S, Satish S. Synthesis of silver nanoparticles by endosymbiont Pseudomonas fluorescens CA 417 and their bactericidal activity. Enzyme and Microbial Technology. 2016; 95: 128-136.
CrossRef - Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumar J, Srinivasan K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochimica Acta Part A: olecular and Biomolecular Spectroscopy. 2011; 79(3): 594-598.
CrossRef - Zare M, Namratha K, Alghamdi S, Mohammad Y. H. E, Hezam A, Zare M, Drmosh, K. Byrappa Q. A, Chandrashekar B. N, Ramakrishna S, Zhang X. Novel Green Biomimetic Approach for Synthesis of ZnO-Ag Nanocomposite; Antimicrobial Activity against Food-borne Pathogen, Biocompatibility and Solar Photocatalysis. Sci. Rep. 2019; 9(1): 8303.
CrossRef - Gola D, Kriti A, Bhatt N, Bajpai M, Singh A, Arya A, Chauhan N, Srivastava S. K, Tyagi P. K, Agrawal Y. Silver nanoparticles for enhanced dye degradation. Current Research in Green and Sustainable Chemistry. 2021; 4: 100132.
CrossRef - Saranya V. T. K, Gowrie U. S. Photo catalytic reduction of methylene blue dye using biogenic silver nanoparticles from the aqueous cladode extract of Casuarina equisetifolia. Indo Am J Pharm Res. 2016; 6:4562–4568
- Vanaja M, Paulkumar K, Baburaja M, Rajeshkumar S, Gnanajobitha G, Malarkodi C, Sivakavinesan M, Annadurai G. Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinorgan Chem Appl. 2014; 2014: 742346.
CrossRef - Joshi S. J, Geetha S. J, Al-Mamari S, Al-Azkawi A. Green synthesis of silver nanoparticles using pomegranate peel extracts and its application in photocatalytic degradation of methylene blue. Jundishapur J Nat Pharm Prod. 2018; 13:e67846
CrossRef - Sumitha S, Vasanthi S, Shalini S, Chinni S. V, Gopinath S. C. B, Anbu P, Bahari M. B, Harish R, Kathiresan S, Ravichandran V. Phytomediated photo catalysed green synthesis of silver nanoparticles using Durio zibethinus seed extract: antimicrobial and cytotoxic activity and photocatalytic applications. Molecules. 2018; 23:3311
CrossRef - Zaban M. I, Mahmoud M. A, AlHarbi M. A. Catalytic degradation of methylene blue using silver nanoparticles synthesized by honey. Saudi Journal of Biological Sciences. 2021; 28(3): 2007-2013.
CrossRef - Miri A, Shahraki Vahed H. O, Sarani M. Biosynthesis of silver nanoparticles and their role in photocatalytic degradation of methylene blue dye. Res Chem Intermed. 2018; 44: 6907–6915.
CrossRef - Roy K, Sarkar C. K, Ghosh C. K. Photocatalytic activity of biogenic silver nanoparticles synthesized using yeast (Saccharomyces cerevisiae) extract. Appl Nanosci. 2015; 5: 953–959.
CrossRef






