Biosorption of Corafix Yellow GD3R using Halimeda gracilis and its Toxicological Assessments
1
Department of Zoology,
Avinashilingam Institute for Home Science and Higher Education for Women,
Coimbatore,
Tamil Nadu
India
Corresponding author Email: poonkothaii_zoo@avinuty.ac.in
DOI: http://dx.doi.org/10.12944/CWE.18.2.30
Copy the following to cite this article:
Yuvanthi P. M, Poonkothai M. Biosorption of Corafix Yellow GD3R using Halimeda gracilis and its Toxicological Assessments. Curr World Environ 2023;18(2). DOI:http://dx.doi.org/10.12944/CWE.18.2.30
Copy the following to cite this URL:
Yuvanthi P. M, Poonkothai M. Biosorption of Corafix Yellow GD3R using Halimeda gracilis and its Toxicological Assessments. Curr World Environ 2023;18(2).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-12-12 |
|---|---|
| Accepted: | 2023-08-28 |
| Reviewed by: | 
 Bozena CZECH
Bozena CZECH
|
| Second Review by: |

 Pratyush Kumar Das
Pratyush Kumar Das
|
| Final Approval by: | Dr. Gangadhar Andaluri |
Introduction
Water is a vital and indispensable requisite for all living organisms because it play a noteworthy role in determining its capability for domestic, agriculture and industrial activities and also accessibility to clean water supply should be available always. Without adequate treatment millions of contaminants are released into water bodies as a results of urbanization, industrial revolution and expanding population which thereby pose water scarcity and pollution to become a major problem1.
One among the leading industry in the economic sector is textiles, where human not only need it for survival, but also evolved into a form of fashion. Almost 1,00,000 diverse categories of dyes are available in the market, among azo reactive dyes occupies about 30% of annual dye material production2. Emission of these complex organic contaminants into the ecosystem is the major cause for the decline of water and soil quality3. When the dye-contaminated waste water is released into aquatic bodies, they deteriorate the water quality and make it unsafe to utilize for domestic tasks and public health4. The occurrence of synthetic dyes in the aquatic ecosystem reduces visibility, affects the growth of fauna and flora and also lowers the photosynthetic rate. They also pose major risk to human health by causing severe impairment to brain, central nervous system, liver, kidney as well as dysfunction of reproductive systemand also cause allergic dermatoses, respiratory illness and malignant tumour5,6. In the current investigation, Corafix Yellow GD3R dye, was less expensive, more productive, and requires less time to color. The chemical groups in these dyes enable them to create covalent bonds with the fabrics, and give them their distinctive properties7. They also have toxic effect, as they decrease the water transparency, and seem to be genotoxic to aquatic plants and animals8. Thus, there is a pressing requisite to spot more sensible, inexpensive and green technology for dye removal before discharging into water bodies to evade destruction of aquatic ecosystem.
Numerous conventional physicochemical techniques have been recommended for the purpose of textile effluent treatment which includes ozonation, membrane filtration, activated carbon adsorption, ion exchange, flocculation, froth flotation, electro coagulation and reverse osmosis9. All these techniques were extremely flexible and beneficial, but because of their high cost and disposable nature, they produce secondary waste that needs to be addressed further. It has been demonstrated that removing color from textile effluent using biological such as microorganisms, algae, seaweed, agriculture wastes and plants are extincing, ecofriendly, economical and an advantageous alternative method10. Indeed, using seaweeds as biosorbent for removing color and other contaminants from dye contaminated waste water is a promising and an appealing environmentally friendly technique. Seaweeds can easily grow in both fresh and saline water depending on the climate, requires less financial input to cultivate and generate least amount of sludge11. H. gracilis was nominated as the candidate seaweed in the current study, because of its strong affinity to remove the dye and the presence of reactive functional compounds on its surface12. Literature reveals that the removal of CYGDR3 using H. gracilis has not been renowned, and therefore the current investigation was formulated to assess its decolorization efficiency against Corafix Yellow GD3R and to evaluate its toxicity against green gram under in vitro conditions.
Materials and Methods
Processing of seaweed and adsorbate for adsorption study
The seaweed was procured from the Mandapam, Tamil Nadu. The acquired seaweed was cleaned in running water, air dried, powdered and stored at room temperature in an airtight container for future investigation. The stock solution was prepared by suspending 1000mg of CYGDR3 dye with 1L of water and the content was left overnight. Proper dilutions were prepared for the study from the stock solution.
Point of zero charge determination
Batch equilibrium approach was performed to calculate the point of zero charge (pHpzc). To a known volume of 0.1M NaCl, the biosorbent was added and the pH (2-11) was regulated with 0.1M HCl/0.1M NaOH. The solution’s initial pH (pHinitial) remained documented, and it was also endlessly stirred at 150 rpm for 24 hours. The solution's pH was determined besides the results recorded as pHfinal. A plot of pH change, pH= (pHinitial - pHfinal), against (pHinitial), was constructed and the biosorbent pHpzc was calculated at the point where pH is zero13.
Decolorization of Corafix Yellow GD3R through batch adsorption experiments
The batch adsorption study for dye removal was performed by varying the dosage of dye and biosorbent concentrations from 100 to 500 mg/L, pH (2-11), incubation time (24-144hrs) and temperature (20-40oC) and the pH was adjusted using 1N HCl/ 1N KOH. The decolorization efficiency of H. gracilis against CYGDR3 was assessed after each incubation period and the decolorization percentage was observed at 580nm using the formula14.

The maximum decolorization percentage was established under optimal conditions and a subsequent study was conducted there as well.
Kinetic and Thermodynamic studies
The kinetic studies was performed at varying time intervals to impart the evidence on the sorption and rate controlling mechanism with Pseudo first order (PFO) and Pseudo second order (PSO), intraparticle diffusion (ID) models respectively. The nature of biosorption at different temperature was studied using thermodynamic factors specifically free enthalpy (G°), total heat (H°) and entropy (S°), below optimized circumstances.
Desorption study
Using the eluents such as NaOH, CH3COOH, HCl and HNO3, at 0.1N concentration separately the efficacy of dye desorption was performed in three consecutive cycles to observe the dye removal from the dye-loaded biosorbent at optimized conditions. The amount of dye desorbed and adsorbed denotes the efficacy of percentage desorption.15
UV-Visible spectral analysis
The spectra of CYGDR3 decolorized with H.gracilis was analyzed through UV-visible spectrophotometer at 300-700nm (Hitachi U2800, Tokyo, Japan) and equated with untreated dye solution to determine the process of decolorization.
Phytotoxicity study
Vigna radiate (Green gram), was selected to demonstrate the toxicity of the CYGDR3 treated and untreated dye solutions. Ten healthy seeds were sown in nine pots separately and kept under room temperature. At regular intervals, the seedlings were watered with treated and untreated dye solutions as well as with tap water which served as control. Each treatment was performed in triplicates. The seedlings were removed and biometric measurements, specifically germination percentage, vigour index, shoot and root lengths were documented on seventh day16.Phytotoxicity index (PI) greater or less than 1, reveals the toxic or stimulatory character of the dye solution exposed to H.gracilis against the seedlings of V. radiata17.

Antimicrobial activity
Corafix Yellow GD3R decolorized with H.gracilis and untreated dye solution were tested against the bacterial (Staphylococcus aureus and Escherichiacoli) and fungal (Aspergillusniger and Rhizopusstolonifer) cultures through agar well diffusion method. On Muller Hinton(bacteria) and Rose Bengal Chloramphenicol (fungi) agar plate’s two wells were bored aseptically and added the test solutions which were kept at 37oC for 24hrs and 28oC for three days respectively. The inhibitory zone circumference (mm) served as index for the pollutant toxicity. The data were statistically determined using mean±SD18.
Results and Discussion
Distinctive characters of Halimeda gracilis
The green macroalgae Halimeda gracilis, belongs to the phylum Chlorophyta and family Halimedaceae (Fig.1). H. gracilis is a surface water species which can flourish by absorbing sunlight and also found in the subtidal zone of southeast coast of Tamil Nadu. The fresh segments H. gracilis was greenish, flattened, subcylindrical, ribbed, extensively calcified, trilobed, infrequently cuneate and the dry segments were pale green to white. They possess a single holdfast to secure them to the substrate and have dichotomous/trichotomous branching19.
 | Figure 1: Dried seaweed of H. gracilis
|
Assessment of zero point of charge (pHzpc) of H. gracilis
It is an crucial aspect in understanding the surface chemistry of the biosorbent20,21. The pHzpc data denotes the pH level at which the biosorbent net charge is zero. The pHzpc of H.gracilis was empirically determined arises at 5.4, as shown in Fig.2. This outcome implies that pH below 7 (acidic) is advantageous for anionic dyes. The research examining how pH influences dye decolorization explored further in the adsorption studies.
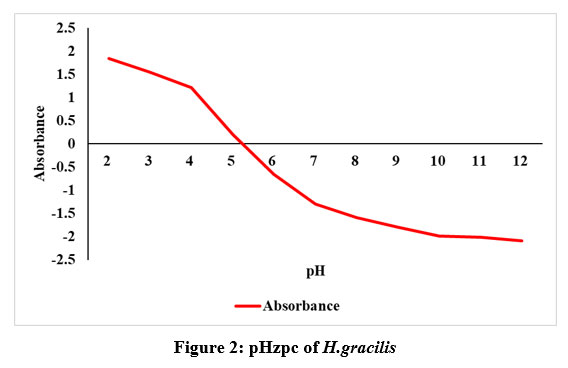 | Figure 2: pHzpc of H. gracilis
|
Batch adsorption studies for Corafix Yellow GD3R decolorization
The process of dye decolorization was assessed through batch mode using diverse adsorption parameters such as concentration of dye and biosorbent, incubation period, pH and temperature. A crucial aspect that influences the adsorption phenomena is the dye concentration which was differ from 100 to 500mg/L by keeping all other factors constant(Fig 3a-3e). The consequence of dye concentration on the removal of CYGDR3 using H. gracilis was intense at 300mg/L (88%) and further rise in dye concentration reveals no substantial effect on decolorization phenomenon (60%). The adsorption sites in the biosorbent are easily occupied by the dye molecules and thereby a significant impact on adsorption capacity was recorded at varying dye concentrations. The concentration of dye offers a stronger dynamic energy, to conquer the diffusive mass transfer barrier involving the biosorbent and adsorbate. Thus at low concentrations, the dyes may interact with the binding sites to promote high removal, whereas rise in dye concentration has no effect on the adsorption phenomena after equilibrium is established because the external surface of the biosorbent gets saturated by the dye molecules. Fucus vesiculosus effectively removed methylene blue and rhodamine B under optimized conditions which supported the findings of the present study22.
The surface of the biosorbent provides binding sites for the dyes to adsorb from aqueous solution23. From Fig.3b, it is evident that the utmost color removal (84%) was observed in dye solution amended with 200 mg/L of biosorbent and its further increase (300-500 mg/L) did not cause any progression in biosorption. The availability of active sites in the biosorbent at low concentration allows the optimum level of dye to adsorb on its surface and thereby increases the dye removal rate24.Whereas, the rate of dye sorption gets declined when the concentration of biosorbent is raised, since they form aggregates at the adsorption site. Thus, the amount of available adsorption sites and the exchange of dye molecules for biosorption depend upon the concentration of biosorbent used in the adsorption process25. According to the results of the present investigation, Ulva lactuca was found to remove methylene blue and malachite green effectively at lower biosorbent concentrations.26.
The entire adsorption process is substantially determined by the dye solution’s pH, since it is vital for the protonation of the dye binding site27.The removal of CYGDR3 using H. gracilis was studied at varying pH (2-11) and it evident from Fig.3c that maximum removal of CYGDR3 was established at pH 6 (88%) and a further raise in the pH (7-11) declined the rate of dye absorption.With regard to point of zero charge, at a low pH, the surface of H.gracilis was protonated (positive charge), which would leads to a powerful electrostatic attraction between the biosorbent (positive surface charge) and CYGD3R (anion) molecules which lead to increase the dye removal. As the pH increases (basic conditions), the surface charge of H.gracilis became negative which might have created electrostatic revulsion amidst the biosorbent and anionic CYGD3R dye molecules which assisted in decreased dye removal28.
Contact time is one of the vital factor for using a biosorbent successfully for practical applications and for quick adsorption29. To evaluate H. gracilis effectiveness at removing dye, the biosorption experiment was conducted over a range of time periods (24–144 hours). It was noticed that the efficacy of dye removal remained high at the early stage and maximum adsorption was found at 72hrs which subsequently falls with no discernible color removal after 72hrs (Fig.3d). Rapid adsorption at the primary stage may be due to the abundance of high number of binding positions for biosorption, but as time proceeds, the remaining binding positions become harder to occupy and takes long time to reach equilibrium because of the repulsion between the biosorbent and adsorbate30. At the initial stage of adsorption physical or ion exchange process may occur at the cell surface and increase the rate of dye removal whereas complexation, precipitation or saturation of binding sites might occur after a lapse of time and thereby results in decreased dye removal31.
The most crucial kinetic variable in the biosorption process is the temperature which has an impact on the adsorption capability of the biosorbent and the mobility of the adsorbate32. An increase in the temperature, increases the dye removal rate and maximum removal was recorded at 25oC (84%) using H.gracilis. Further increase in temperature (30oC -40oC) decreases the dye removal efficiency (Fig.3e), which might be initiated by the modification in the texture of the biosorbent or material deterioration33. Ulva lactuca removed methylene blue to the greatest extent at the ideal temperature of 25oC which supported the results of the present investigation34. According to the temperature profile, the sorption capacity rises with temperature until it reaches a maximum and then falls. The decline in dye removal after rise in the optimal temperature may be related to denaturation, which occurs when a biosorbent is exposed to extremely high temperature which damages the active binding sites or the tendency to desorb dyes from the interface of the solution35.Thus at optimal conditions (Dye concentration-300mg/L, pH 6, Biosorbent concentration - 200mg/L, Temperature - 25o C,Incubation time - 72 hrs), 88% of CYGD3R has been removed by H.gracilis.
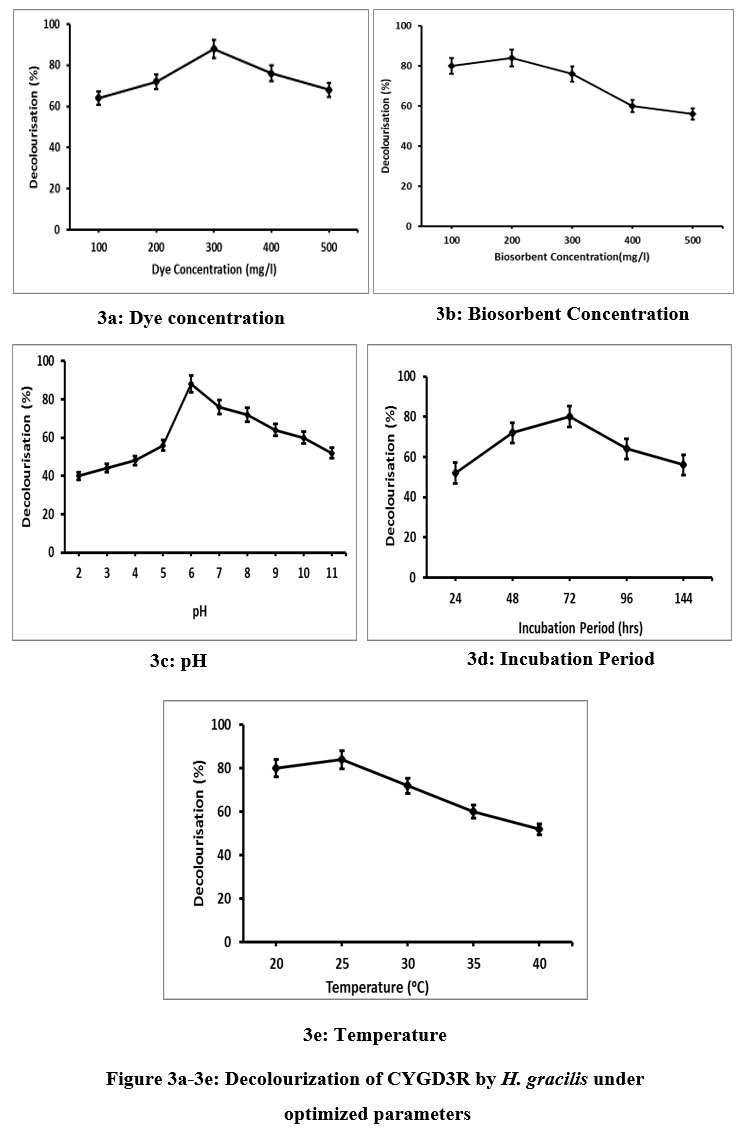 | Figure 3a-3e: Decolourization of CYGD3R by H. gracilis under optimized parameters.
|
Adsorption kinetics and thermodynamic studies
Adsorption kinetic models namely the PFO, PSO and ID remained assessed to demonstrate of possible physiochemical interactions involved between the biosorbent and dye molecule during adsorption process. The kinetic model's linear equations were presented below,
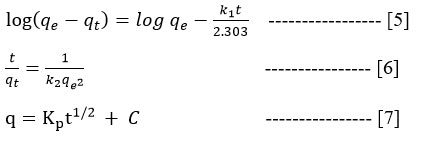
where the adsorption level constants are k1 and k2(mgming-1), qe and qt(mgg-1) represent the quantity of dye biosorbed at the equilibrium at given time (t), q indicates sum of dye adsorbed (mgg-1), t1/2 is the square root of time t (min),Kp stands for intraparticle diffusion rate constant and the interceptis denoted as C. The datas of k1, k2 and qe are equated from log (qe – qt) vs t and t/qt vs t polts separately and their findings were depicted in Table 1 and picturized in Fig.4a and 4b.The R2 value (0.011) of PFO was less than the PSO (R2> 0.95) kinetic models which proves the later to be more suitable. This further implies that the process of adsorption occurs through chemisorption concerning the equilibrium armed forces through the exchange of electrons among the biosorbent and adsorbate36. The adsorption results of the present study obey pseudo-second kinetics model which agrees with the reports on the removal of yellow dye using Padina pavonica37. Fig.4c depicts that the linear form do not passes through the origin and proves the ID is not the adsorption rate controlling stage for the CYGD3R adsorption on to H.gracilis.
The thermodynamics factors such as free enthalpy (G°), total heat (H°) and entropy (S°) were evaluated to study the spontaneity and the adsorption of CYGD3R on to H.gracilis using the following equations

where,KD (thermodynamic equilibrium constan)t, R (universal gas constant -8.314 J mol-1 K-1) and T (temperature). The Go and Ho datas existed attained from the slope and intercept of Van’t Hoff plot of experimental data on lnKDvs 1/T (Fig. 4d) and their results were depicted in Table 1. The negative datas of G° show that the biosorption of CYGD3R onto H. gracilisis a spontaneous and feasible manner and the positive datas of H° and S° represents the endothermal in nature and rising randomness at adsorbate-biosorbent interface during the biosorption procedure. The rise of randomness on solid-liquid interface in the sorption system throughout the adsorption method is shown by the positive value of variation in entropy (S°). Similar such endothermic, feasible and spontaneity was noticed in Direct Red 81 removal by Argemone mexicanawere38.
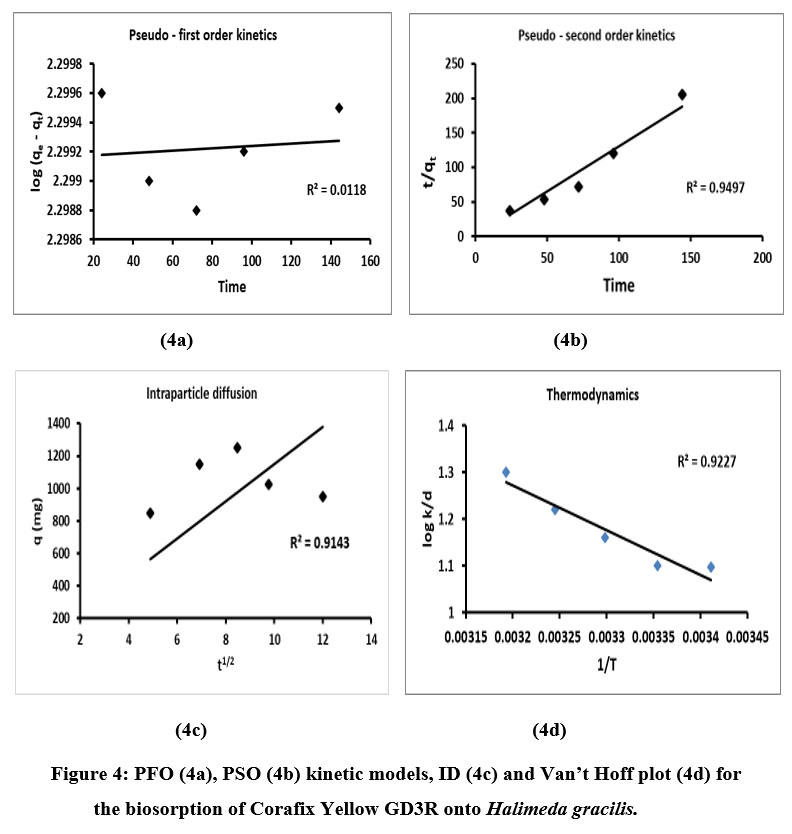 | Figure 4: PFO (4a), PSO (4b) kinetic models, ID (4c) and Van’t Hoff plot (4d) for the biosorption of Corafix Yellow GD3R onto Halimeda gracilis.
|
Table 1: Adsorption kinetics and thermodynamic parameters
Adsorption kinetics parameters | |||||||||||
PFOkinetics*
| PSO kinetics* | ID** | |||||||||
qe | K1 | R2 | qe | K2 | R2 | Kp | C | R2 | |||
2.29 | 7.88 | 0.01 | -12.94 | 1.43 | 0.94 | 6.028 | 994.27 | 0.91 | |||
Thermodynamics | |||||||||||
Temperature (oC) | Go***
| Ho***
| So
| R2 | |||||||
20 | -182 |
4.34 |
9.59 |
0.92 | |||||||
25 | -224 | ||||||||||
30 | -285 | ||||||||||
35 | -350 | ||||||||||
40 | -432 | ||||||||||
*(mg min g-1), ** (mg g-1 min-1/2), ***(kJ mol-1), ****(J mol-1K-1)
Corafix Yellow GD3R desorption using different eluents
The biosorbent needs to be recycled to make the biosorption process a profitable one. Desorption, a highly efficient chemical procedure was utilized for recycling the dye-loaded biosorbent, since it does not damage the biosorbent structure39. Under optimal conditions, three successive desorption cycles were employed using the selected eluents. Among the eluents employed, 0.1N HNO3 seems to desorb 60% of the dye from the biosorbent, followed by others in the first cycle and thereafter a progressive decrease was detected in the second and third cycles (Fig.5). For industrial applications, the recovery of dye from the chosen biosorbent is crucial, since it must not only have great biosorptive potential, but also be easy to use economically, simple to regenerate and reuse. Therefore, the eluent, kind of biosorbent, and biosorption mechanism should be the fundamental requirement for a proper desorption process40.Hence the choice of desorbing solvent or eluent is crucial for optimal regeneration and is greatly dependent on the kind of biosorbent and the biosorption mechanism41.
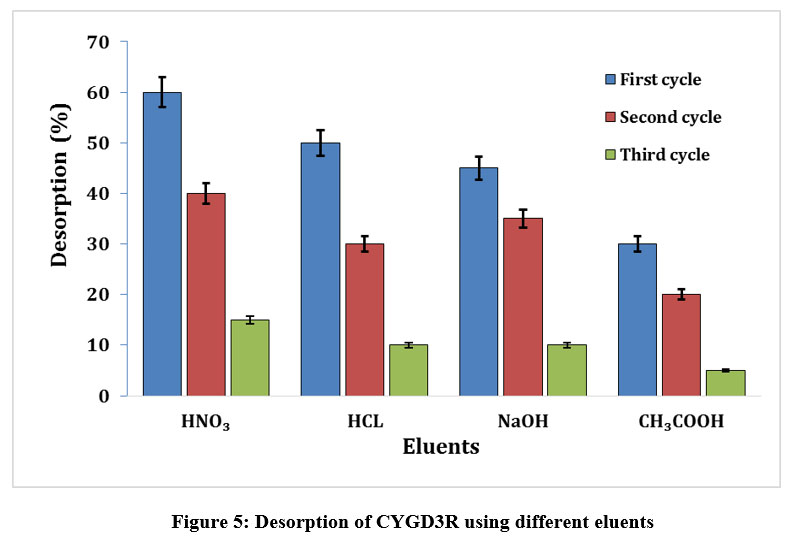 | Figure 5: Desorption of CYGD3R using different eluents
|
UV-visible spectral analysis
The UV-visible spectrum of the CYGD3R concealed a peak at 580 nm and it was completely disappeared in the treated dye solution. The decrease in absorbance peak in the spectrum provides sturdy evidence for the decolorization of the dye (Fig.6). The diminished or disappearance of the peak may be due to the enzymatic biodegradation of dyes or by the breakdown of chromophores42. Similar such decrease/ disappearance in the UV-visible absorption peak was observed when methylene blue was exposed to Arthrospira platensis which may be either due to adsorption or biodegradation43.
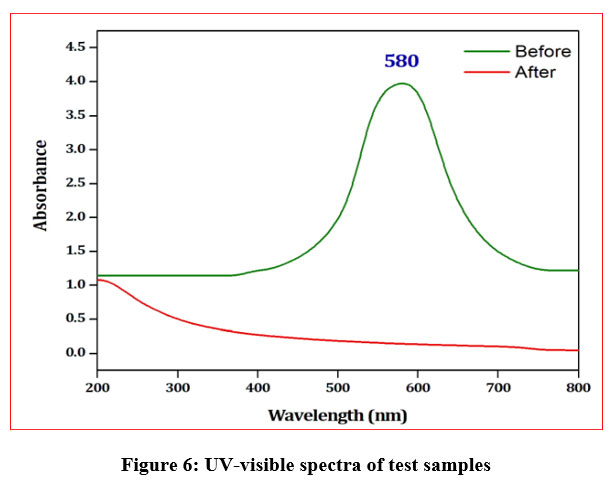 | Figure 6: UV-visible spectra of test samples
|
Phytotoxicity study
When untreated textile dyeing effluent was released into the terrestrial ecosystem or utilized for agriculture, they cause harmful effects to biota. Subsequently, measuring their toxicity before and after decolorization was fraught with concern. Vigna radiata (Green gram) was used as the candidate plant to evaluate the toxicity of CYGD3R treated with H.gracilis and was compared untreated with tap water (control). The results of the biometrics revealed that the dye-treated solution enhanced the percentage of germination (93%), shoot (5.85 cm) and root (2.59 cm) length and vigour index (785) when considered with untreated dye solution (67%, 4.33cm, 1.57cm and 395) whereas the seedlings grown with the tap water (100%, 6.36cm, 3.1cm and 946) showed a positive impact. The phytotoxicity index was documented as 0.16, which is less than 1 thereby demonstrating the stimulatory effect of the treated of dye solution and thereby proves its nontoxic nature. Similar such decreased in seedling growth was observed in Triticum sp. seeds watered with untreated red and blue dye solution where the dyes may interact with the root and quickly penetrate into the embryonic axis membrane44.Also, the reduced shoot and root length of Lemna minor seedlings exposed to untreated solution cause harmful effects such as reduced enzyme activation, water imbalance, nutritional deficiencies, hormonal changes that affect membrane permeability and plant growth45.
Microbial toxicity studies
The results revealed that S. aureus and E.coli exhibited 6 mm and 8 mm zone of inhibition and Aspergillus niger and Rhizopus stolonifer recorded in 9mm and 11mm zone of inhibition against untreated dye solutions. In treated dye solution, there was no discernible growth inhibitory zone, indicating that the degraded dye was not harmful to the examined bacterial and fungal isolates.
Conclusion
The present study investigates the potentiality of Halimeda gracilis in Corafix Yellow GD3R removal under in vitro conditions. Corafix Yellow GD3R dye adsorption was found to be strongly reliant upon the concentration dye and biosorbent, pH, incubation time and temperature. Under optimized conditions, H.gracilis removed 88% of Corafix Yellow GD3R dye. The adsorption monitored pseudo second order kinetics and thermodynamic factors exhibited the adsorption of dye on top of H. gracilis remained spontaneous, feasible and endothermic in nature. Thus from the obtained outcomes, it can be accomplished that for the textile wastewater comprising variety of azo reactive dyes, H. gracilis can be used as a potent biosorbent under optimized conditions due to its great biosorption efficiency, reusability and desire to serve as the foundation for the creation of ecofriendly pollution-free environment.
Acknowledgement
The authors are sincerely thankful to the authorities of Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore, Tamil Nadu, India for providing research facilities.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Sources
This research did not receive any specific grant from funding agencies in public, commercial, or not-for-profit sectors.
References
- Lebron Y.R, Moreira V.R, Santos L.V. Studies on dye biosorption enhancement by chemically modified Fucus vesiculosus, Spirulina maxima and Chlorella pyrenoidosa Algae. J. Clean. Prod.2019;240: 118197.
- Jadhav J.P, Phugare S.S, Dhanve R.S, Jadhav S.B. Rapid biodegradation and decolorization of Direct Orange 39 (Orange TGLL) by an isolated bacterium Pseudomonas aeruginosa strain BCH. Biodegradation. 2010;21(3):453-63.
- Kroumov D. Determination of the mass transfer-limiting step of dye adsorption onto commercial adsorbent by using mathematical models. Environ. Technol. 2014; 35: 2356-2364.
- Robinson T, Mullar G, Marachant R. Remediation of dyes in textile dyeing industry effluents a critical review on current treatment technologies with a proposed alternative. Environ. Technol. 2001; 77: 247-255.
- Salleh M.A, Mahmoud D.K, Abu N.A, Karim W.A, Idris A. Methylene blue adsorption from aqueous solution by langsat (Lansium domesticum) peel. J Purity, Utility Reac and Envi. 2012; 1: 472-495.
- Wilkinson S. M, Mc Gechaen K. Occupational allergic contact dermatitis from reactive dyes. Contact Derm. 1996; 35: 376.
- Senturk T, Caml? C, Yildiz S. Comparing the phytoremediation efficiency of three different algae for the nutrient removal of Gediz River in Manisa/Turkey. CBU Journal of Science .2017; 13(3):737-743.
- Gonçalves B.B, Giaquinto P.G, Silva D.S, Meloe C, Neto S, Lima A.S, BritoDarosci A. A, Portinho J.L, Carvalho W.F, Rocha T.L. Ecotoxicology of glyphosate-Based Herbicides on Aquatic Environment. J. Biochem. Technol. 2019; 55-71.
- Wang J.L, Chen C. Biosorbents for heavy metals removal and their future- A review. Biotechnology Advances.2009; 27: 195–226.
- Nilson T, Peterson U. Successional reflectance trajectories in northern temperate forests. Int. J. Remote Sens.1993; 14(3):609-613.
- Ozer A, Ozer D. Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto Saccharomyces cerevisiae: determination of biosorption heats. J. Hazard. Mater. 2003; 100: 219-229.
- Varma R, Turner A, Brown M.T. Bioaccumulation of metals by focus ceranoides in estuaries of South West England. Mar. Pollut. Bull. 2011; 62 : 2557–2562.
- Hashem, A., Aniagor, C. O., Morsy, O. M., Abou-Okeil, A and Aly, A. A. Apricot seed shell: An agro-waste biosorbent for acid blue193 dye adsorption. Biomass Conversion and Biorefinery. 2022.
- El-Sheekh M.M, Gharieb M.M, Abou-El-Souod G.W. Biodegradation of dyes by some green algae and Cyanobacteria. Int. Biodeterior. Biodegradation.2009; 63: 699-704.
- Phugare S.S, Kalyani D.C, Patil A.V, Jadhav J.P. Textile dye degradation by bacterial consortium and subsequent toxicological analysis of dye and dye metabolites using cytotoxicity, genotoxicity and oxidative stress studies. J. Hazard. Mater.2011: 186, 713-723.
- Akar S.T, Akar T, Cabuk A. Decolourisation of a textile dye, reactive red 198 (RR198) by Aspergillus parasiticus fungal biosorbent. Braz. J. Chem. Eng.2009; 26: 399-405.
- Al-Tohamy R, Sun J, Fareed M. F, Kenawy E. R, Ali S. S. 2020. Ecofriendly biodegradation of Reactive Black 5 by newly isolated Sterigmato myceshalophilus SSA1575, valued for textile azo dye wastewater processing and detoxification. Scientific reports.2020; 10: 1-16.
- Mekki A, Dhouib H, Sayadi S. Polyphenols dynamics and phytotoxicity in a soil amended by olive mill wastewaters. J. Environ. Manage.2007; 84:134- 140.
- Saratale R.G, Saratale G.D, Chang J.S, Govindwar S.P. Decolorizationandbiodegradation of reactive dyes and dye wastewater by a developed bacterial consortium. Biodegradation.2010: 21: 999-1015.
- Yadav M, Thakore S, Jadeja R. Removal of organic dyes using Fucus vesiculosus seaweed bioadsorbent an ecofriendly approach: Equilibrium, kinetics and thermodynamic studies. Environmental Chemistry and Ecotoxicology. 2022; 4, 67–77.
- Berber-Villamar N. K, Netzahuatl-Muñoz A. R, Morales-Barrera L, Chávez-Camarillo G. M, Flores-Ortiz C. M, Cristiani-Urbina E. Corncob as an effective, eco-friendly, and economic biosorbent for removing the azo dye Direct Yellow 27 from aqueous solutions. PLoS ONE. 2018: 13, e0196428.
- Xing X, Jollanda M.H, Huisman J. Competition between cyanobacteria and green algae at low versus elevated CO2: who will win, and why?.Journal of Experimental botany.2017: 68(14): 3815–3828.
- Yadav M, Thakore S, Jadeja R. Removal of organic dyes using Fucus vesiculosus seaweed bioadsorbent an ecofriendly approach: Equilibrium, kinetics and thermodynamic studies. J. Environ. Chem.Ecotoxicol.2022; 4:67-77.
- Yagub M.T, Sen T.K, Afroze S, Ang H.M. Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid Interface Sci. 2014 209: 172–184.
- Azmat R. Marine green algae Codiumyengarii as a good biosorbent for elimination of reactive black 5 from aqueous solution. Pak. J. Pharm Sci. 2014; 5, 1443-1449.
- Dharajiya D, Shah M, Bajpai B. Decolorization of Simulated Textile Effluent by Phanerochaete chrysosporium and Aspergillus fumigatus A23. Nat. Environ. Pollut. Technol.2016; 15(3).
- Deokar R, Sabale M. Biosorption of Methylene Blue and Malachite Green from Binary Solution onto Ulva lactuca. Int. J. Curr. Microbiol. Appl. Sci.2014: 295-304.
- Das N, Charumathi D. Remediation of synthetic dyes from wastewater using yeast-an overview.Indian J. Biotechnol.2012; 11: 369-380.
- Ramachandran P, Sundharam R, Palaniyappan J, Pudukkadu A. Potential process implicated in bioremediation of textile effluents: A review.Adv. Appl. Sci. Res.2013; 4(1):131-145.
- Loong Y, Lewis W. L. 2013. The disappearing Environmental Kuznets Curve: A study of water quality in the Lower Mekong Basin (LMB). J. Environ. Manage.2013; 131:415-425.
- Idris M.N, Ahmad Z. A, Ahmad M.A. Adsorption equilibrium of malachite green dye onto rubber seed coat based activated carbon. Fudma J. Sci.2011; 3: 67-76.
- Chiou M. S, Ho P. Y, Li H. Y. Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes and pigm.2004; 60(1), 69-84.
- Arulselvi I.P, Barathi S. Decolourization, degradation, and toxicological analysis of textile dye effluent by using novel techniques – Review. Int. J. Sci. Res.2015; 3:2118- 2136.
- Nacèra Y, Aicha B. Equilibrium and kinetic modelling of methylene blue biosorption by pretreated dead Streptomyces rimosus: Effect of temperature. Chem. Eng. J.2006; 119(2-3):121-125.
- Abdelwahab O, El Sikaily A, Khaled A, Nemr A. Removal of Methylene Blue from aqueous solution by marine green alga Ulva lactuca. J.Chem. Ecol. 2006; 22:2, 149-157.
- Alqaragully M.B (2014) Removal of textile dyes (maxilon blue and methyl orange) by date stones activated carbon. Int. J. Adv. Res. Chem. Sci. 2014; 1(1): 48-59.
- Hameed B.H. Spent tea leaves: A new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J. Hazard. Mater.2009; 161: 753-759.
- Fakhry E.M. Padinapavonica for the removal of dye from polluted water. AmJ Plant Sci.2013; 4, 1983-1989.
- Khamparia S, Jaspal D. Adsorptive removal of direct red 81 dye from aqueous solution onto Argemonemexicana. Sustainable Environment Research. 2016;26(3): 117-123.
- Kwak H.W, Kim M.K, Lee J.Y, Yun H, Kim M.H, Park Y.H. Preparation of bead type biosorbent from water soluble Spirulina platensis extracts for chromium (VI) removal, Algal Res. 2015; 7:92-99.
- Chen S, Zhang J, Zhang C, Yue Q, Li Y, Li E. Equilibrium and kinetic studies of methyl orange and methyl violet ad sorption on activated carbon derived from Phragmites australis. Desalination.2010; 252, 149-156.
- Das C, Gupta S. De S. Treatment of dyeing effluent from tannery using membrane separation process, Int J Environ Waste manag.2010; 5: 354-367.
- Bagchi M, Ray L. (2015) Adsorption behavior of reactive blue 4, a tri-azine dye on dry cells of Rhizopusoryzae in a batch system. Chem. Speciation and Bioavailability.2015; 27(3):112-120.
- Mansour AT, Alprol AE, Abualnaja KM, El-Beltagi HS, Ramadan KM, Ashour M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. J Mater. 2022; 31:15(11):3922.
- Brahmbhatt NH, Jasrai RT. The role of algae in bioremediation of textile effluent. Methods. 2016; 21:28.






