Pesticide Soil Pollution: An Overview About Advantages and Disadvantages of Different Remediation Technologies
Puspendu Shit1
 , Indranil Bhattacharjee14
, Indranil Bhattacharjee14
 , Partha Pratim Chakravorty1
*
, Partha Pratim Chakravorty1
*
 , Harekrishna Jana2
, Harekrishna Jana2
 and Yuji Sakai3
and Yuji Sakai3

1
Department of Zoology,
Raja Narendra Lal Khan Women’s College (Autonomous),
Paschim Medinipur,
West Bengal
India
2
Department of Microbiology,
Raja Narendra Lal Khan Women’s College (Autonomous),
Paschim Medinipur,
West Bengal
India
3
Department of Environmental Chemistry and Chemical Engineering,
Kogakuin University,
Tokyo,
Japan
4
Department of Zoology,
Dr Bupendra Nath Dutta Smriti Mahavidyalaya,
Hatgobindapur,
West Bengal
India
Corresponding author Email: parthapratimchakravorty@yahoo.in
DOI: http://dx.doi.org/10.12944/CWE.18.2.25
Copy the following to cite this article:
Shit P, Bhattacharjee I, Chakravorty P. P, Jana H, Sakai Y. Pesticide Soil Pollution: An Overview About Advantages and Disadvantages of Different Remediation Technologies. Curr World Environ 2023;18(2). DOI:http://dx.doi.org/10.12944/CWE.18.2.25
Copy the following to cite this URL:
Shit P, Bhattacharjee I, Chakravorty P. P, Jana H, Sakai Y. Pesticide Soil Pollution: An Overview About Advantages and Disadvantages of Different Remediation Technologies. Curr World Environ 2023;18(2).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-03-08 |
|---|---|
| Accepted: | 2023-06-13 |
| Reviewed by: | 
 Ashtari
Ashtari
|
| Second Review by: |

 Bhavana Tomar
Bhavana Tomar
|
| Final Approval by: | Dr. Lam Sze Mun |
Introduction
Pesticides are chemicals used to reduce, remove, prevent, or destroy any pest.Insecticides, fungicides, herbicides, molluscicides, nematicides, bactericides, piscicides, rodenticides, avicides, animal repellents, antimicrobials, and soil fumigants are examples of pesticides1. Pest infestation destroys approximately 45% of annual food production2. Pesticide use helps to keep pests away from crops and can improve crop yield and quality1.In modern times, there are more than 500 substances that are authorized and utilized globally as pesticides or their derivatives. Following the conclusion of the Second World War, the application of pesticides in the farming sector has experienced a steady escalation, resulting in heightened food production worldwide. Among the nations in South Asia, India is the most significant consumer of pesticides, accounting for 3% of global usage in the protection of crops. Organophosphates, organochlorins, and neonicotiniods are some of the most commonly employed pesticides in India3.
However, due to their unscientific and excessive application, 80 to 90% of pesticides applied reach organisms other than their target organism and are deposited on non-target soil and water, contributing to agro-ecosystem pollution3,2.Pesticides possess the capability to disturb the functioning and composition of the ecosystem as they enter the food chain and have adverse effects on the biotic elements of the ecosystem, including soil organisms, plants, animals in the wild, aquatic creatures, and domesticated animals.The widespread presence and enduring impact of diverse pesticides and organic pollutants derived from agriculture have caused significant harm to humanity due to their ability to accumulate in living organisms and their high levels of toxicity. These pesticides have been observed to disrupt the proper functioning of the endocrine and reproductive systems in various organisms. Specific pesticides such as dichlorodiphenyltrichloroethane (DDT), chlordane, aldrin, dieldrin, endrin, mirex, heptachlor, and hexachlorobenzene have detrimental effects on both human health and the environment2.According to the Indian Council of Medical Research (ICMR), approximately 1 million lives are lost annually worldwide due to the persistent effects of pesticide poisoning, resulting in long-term illnesses3.
Numerous techniques for remedying the presence of pesticides in soil have been devised and put into practice, with the aim of eradicating, lessening, and segregating them.However, remediation through the separation and destruction of soil contaminants is time-consuming and costly4. Physical, chemical, and biological methods are the three main approaches used in the separation and destruction of contaminants, depending on the contaminants' characteristics, soil porosity, soil pH, and so on5.The selection of the remediation technique for a polluted site is based on factors such as the nature and concentration of pollutants, soil type, and properties, climate conditions, regulatory obligations, the presence of additional contaminants, as well as cost and time constraints6.
As a result, this study covers the procedure, benefits, and drawbacks of the physical, chemical, and biological techniques that are currently accessible for the restoration of pesticide-polluted soil.
Physicochemical Methods
Immobilization Technologies
The prevention of soil contaminant migration from waste is achieved through immobilization techniques. These techniques include containment methods, solidification/stabilization methods, and vitrification methods, which are the three primary approaches utilized7.
Containment-immobilization
The primary goal of containment is to prevent or control the leakage or leaching of contaminated liquids or semi-liquids into non-contaminated areas. Pumping, draining, capping, and the installation of slurry walls are all basic containment techniques7.
There are several kinds of containment technology. They are broadly classified as active andpassive methods. Slurry walls were built in the field using settlement plates, vane shear, and earth pressure cells. Within a few days, shear strength increases and permeability decreases, preventing contaminants from leaking from the containment zone8. A soil comprised of clay and the presence of octadecyltrimethyl ammonium bromide, a cationic surfactant, as well as reactive barriers modified with kaolinite, montmorillonite, kaolinite, and palygorskite clay minerals, were all subjected to leaching and permeability experiments.More than 85% of the added pesticide compound was washed away through the unaltered natural clay barrier in the soil9.
Containment immobilization is not a true remediation technique; it simply keeps contaminants from leaking into the surrounding environment. Additional chemical and biological techniques are applied to remediate containment contaminants. The technique of containment immobilization has found extensive application in addressing soils that are severely contaminated. Because applied containment technologies are not completely satisfactory, they require close supervision and continuous monitoring10.
Solidification
The process of solidification is used to remediate toxic waste or highly polluted soils. The foundation of this method is the solidification or decrease in the mobility of pollutants, the majority of which are heavy metals. Preventing polluted items from endangering the environment is the aim11. The processes of solidification and stabilization are distinct. Stabilization is a chemical process that converts hazardous waste materials into less harmfulsubstances,and solidification is the process of converting waste materials to solid or semi-solid formsto reduce the permeability or leaching of contaminants7.
During a heat stability experiment, a total of 33 pesticide combinations, comprising 32 insecticides and 4 additional pesticides, were subjected to examination. The study involved placing the samples on a thermostat at 54°C for 14 days. The findings indicate that the majority of the active ingredients in the pesticide mixtures displayed reduced stability compared to their individual formulations12. Contaminants containing low-volatile organics can be managed through stabilization. The effectiveness of solidification/Stabilization technologiesislimited for pesticide remediation6.
Vitrification
Vitrification is a thermal decontamination technique that converts polluted soil into a stable vitreous product.The process of vitrification involves turning toxic waste into items that resemble glass.As per the Environmental Protection Agency of the United States, it is the "best demonstrated available technology" for heavy metals and radioactive waste. Nevertheless, it stands as the costliest method for immobilization7.
Ex-situ and in-situ vitrification are both possible. Graphite electrodes are inserted into the soil during in-situ vitrification to generate a high electric current, while the high temperature (over 1,700oC) melts the soil into a molten block13.
According to various reports, immobilization technologies were used to remediate pesticide-contaminated soils such as Dichlorodiphenyltrichloroethane (DDT), Dichlorodiphenyldichloroethylene (DDE), Dieldrin, Terbuthylazine, Carbofuran, chlorpyrifos, Diuron, Atrazine, and others. A 16-foot-deep trench was created to treat about 305 m3 of contaminated soil using in-situ vitrification. Pretreatment concentrations of 4,4-DDT and Dieldrin were 13000 and 4600 g/kg, respectively. Both pesticide concentrations were reduced to less than 16 g/kg after in-situ vitrification14.
Separation Technologies
Separation technologies are used when contaminants in soil are recalcitrant and persistent, making them less accessible to other destructive remediation methods.
Soil washing
This technique involves the dissolution or suspension of contaminated soils in a solution of water. This method eliminates contaminants from the soil by transferring them from bigger soil particles to the liquid phase(Figure 1). The optimal conditions for soil washing include wash or rinse temperature, surfactant concentration, and pH15. Soils must contain at least 50% sand and gravel to be suitable for soil washing10.
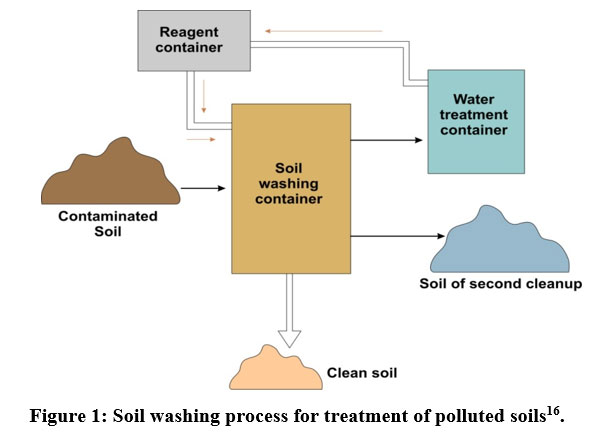 | Figure 1: Soil washing process for treatment of polluted soils16.
|
Solvent extraction
Ex-situ solvent extraction separates contaminants from soil by applying high-shear energy and dissolving them in organic solvent solutions. The supercritical fluid extraction (SFE) method separates various contaminants such as pesticides, phenols, and hydrocarbons. Methanol is frequently used as the primary solvent in this method, along with carbon dioxide (CO2). By channeling CO2 through the soil, contaminants are solubilized in methanol and collected for disposal17. Subcritical water extraction (SCWE) was used to extract parathion, diazinon,and phenthoate from polluted soil. The extraction efficiency was 99.9%, and the final pesticide concentration was less than 0.5 mg/kg at 150°C and 2 MPa. The water flow rate was 0.5 mL/min, and the total extraction time was 20 minutes18. Solvent extraction remediation studies were conducted on soil contaminated with p,p'-DDD, p,p'-DDE, p,p'-DDT, and toxaphene. Methanol was utilized as the solvent, maintaining a ratio of 1.6 parts methanol to soil.This solvent extraction technique reduces pesticide concentrations in soil by more than 99% while also reducing the amount of material required for further extraction by 25%19.
Solvent extraction is currently commercially available for pesticide-contaminated soils. The prime drawback of utilizing this methodology is the exorbitant expense associated with ex-situ implementation.In addition, a certain level of pressure is maintained to keep the solvent liquid and prevent vaporization as the temperature rises. Low permeability soils necessitate high-pressure solvent extraction over a longer time period15.
Surfactants
Surfactants that reduce aqueous solution surface tension are used to solubilize soil contaminants4. The formation of a micelle with a hydrophobic core and a hydrophilic surface is possible with a surfactant that contains both hydrophobic and hydrophilic components.By segregating hydrophobic organics into a hydrophobic core, the surfactant elevates its water solubility.Surfactants showed variable degrees of solubilization of hydrophobic organic compounds depending on their electrical characteristics, polarities, HLB numbers, and CMC values20. Industrially synthesized synthetic surfactants include sulphonates, Brij 35, ethoxylated alcohols, Triton, and sodium dodecylbenzene sulphonate (SDBS). Surfactant and absorbent were used to treat the soils of two sites contaminated with chlordane, DDT, and Mirex. As a surfactant, Triton X-100 was used, and activated carbon was used as an absorbent. According to the findings, triton X-100 improves soil washing and contaminant adsorption by activated carbon21. In another study, surfactant concentration, pH, and ionic strength were studied as potential factors in pesticide removal. For the removal of 2,4-dichlorophenoxyacetic acid (2,4-D) from contaminated soils, two surfactants, sodium dodecyl sulfate (SDS) and ethoxylated lauryl ether (Brij 30) were used. Up to 50% and 80% of 2,4-D were removed in a single wash and two continuousitems of washings with SDS, respectively, whereas Brij 30 removes only 13% of 2,4-D in optimal conditions. The optimal SDS and Brij 30 concentration was 5g/L. The extraction efficiency is greatest when the pH is close to neutral. Even a small pH change to 8 resulted in a significant reduction in pesticide extraction. Brij 30's percentage of extraction was shown to increase when sodium chloride was used to change the ionic strength of the extraction mixture. The addition of 1% NaCl increased extraction from about 12% when no NaCl was added to 30%, whereas no such effect was observed in the case of SDS22.
Biosurfactants, such as rhamnolipids, are more environmentally acceptable for pesticide-contaminated soil remediation because they are compatible with and beneficial to the soil environment. The drawback of synthetic surfactants is that they are hazardous to the soil microbial population and challenging to remove from the soil due to the development of high-viscosity emulsions and limited water solubility10.
Cyclodextrins
Cyclodextrins are used to remove pesticides from contaminated soil as non-toxic alternatives to surfactants and organic solvents. Cyclodextrins entrap many organic compounds in their structural ring due to a low polarity cavity with a small and stable molecular structure. Cyclodextrin can thus solubilize a wide range of organic contaminants23. Many studies have found that cyclodextrins' nanoporouscarbon structure is effective at removing pesticides such as DDT, Dichlorodiphenyldichloroethane (DDD), and DDE5.An ex-situ soil wash study was conducted to extract soil pollutants using carboxylmethyl—cyclodextrin. The removal efficiency for organochlorine pesticides was 94.7% with two continuous soil items ofwashings at 60oC temperature and 40 kHz ultrasonication in 50 mL L-1 maize oil for 20 minutes, 87.2% for mirex, 98.5% for endosulfans, and 92.3% for chlordane24. In a study, 25 g/L of methylcyclodextrin as well as 100 ml / L of sunflower oil at 50 ° C and 35 kHz for 30 minutes removed approximately 99% of organochlorine pesticides (OCP), DDT, endosulfans, heptachlor, and chlordane from solution25.
Although all cyclodextrin research has been conducted on a laboratory scale, pesticides that form strong inclusion complexes can be removed by using a low cyclodextrin concentration. Higher amounts of cyclodextrins should be used in soil with high pesticide contamination. The findings suggest that cyclodextrins could be effective in cleaning up pesticide-contaminated soils10.
Soil flushing
Soil-flushing is a method of treatment in which a flushing solvent is introduced into or applied to the contaminated site's surface(Figure 2). Despite the paucity of studies in this area, chemically enhanced flushing can effectively remove a variety of contaminants26. This method can be used to remove radioactive materials, inorganic chemicals, metals, organic compounds, and inorganic compounds.To improve the efficiency of this technique, appropriate additives are used. This process's sludge can be reused by mixing it with soil or by further treatment with solvent extraction, solidification, or vitrification and then mixing it with soil. Another remediation technology must be used to treat the contaminated soil to reduce the amount of material; this technique is often used as a pre-treatment13.
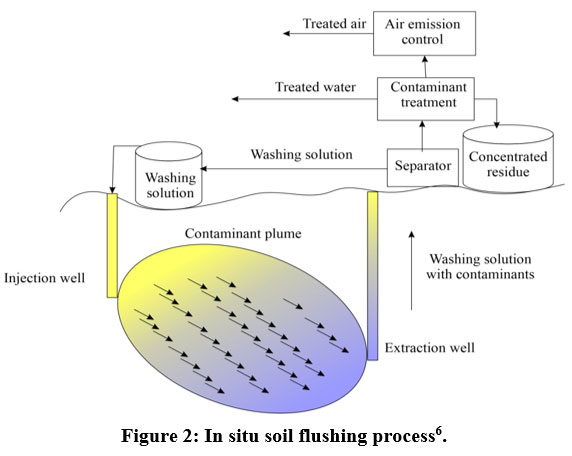 | Figure 2: In situ soil flushing process6.
|
A medium organic content soil was artificially contaminated with phosalone, and an ethanol aqueous solution was used to flush the soil. 99% phosalone extraction was achieved by using a flushing solution containing 10% ethanol by volume 27. Sixteen different solvents were used to flush contaminated soil with hexachlorocyclohexane (HCHs), DDTs, chlordane, and mirex. Ethyl acetate is the most effective at removing HCHs (87.6%) and DDTs (86.9%). And the organic solvents' chlordane removal efficiency was 70% with petroleum ether and 63.5% with mirex and propanol28.
Other techniques, such as electrokinetic methods, activated carbon, and biodegradation, can be combined with soil flushing. Because soil flushing is performed on-site, there is no need to excavate, handle, or transport polluted soil to the treatment area.As a result, the study's cost is reduced. However, soil flushing necessitates the use of additional technology to completely remove contaminants from soils6.
Oxidative process
Advanced oxidative processes, among other methods, have great potential for soil remediation and can be applied as a pre- or post-treatment in several other investigations.The pollutantsare degraded using this technique by mineralizing into inorganic compounds, water, carbon dioxide, or even inert components17.Pesticides, polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and total petroleum hydrocarbons (TPHs) are remedied from contaminated soil using advanced oxidative processes (AOPs)29.Five types of advanced oxidative processes including Fenton oxidative processes, plasma oxidation, ozonation, TiO2 photocatalysis, and persulfate oxidation are discussed here.
Fenton Advanced Oxidation Processes
The advanced oxidation process in Fenton is the oxidation of iron ions (Fe2+) to hydrogen-containing mediums (H2O2), generating a reactive hydroxide atom (•OH), and acts by oxidizing organic contaminants toward less harmful byproducts17 (Figure 3).This technique can benefit from the use of light, electrical current, and ultrasound. The electrical current generates in situ hydrogen peroxide by reducing O2 in the presence of Fe2+, which avoids the continuous addition of hydrogen peroxide. Hydroxyl radicals are produced in the photo-Fenton process by activation of iron (III) alone or with hydrogen peroxide5.
 | Figure 3: Photo–Fenton degradation of pesticides30.
|
Although the Fenton oxidation process is effective in eliminating pollutants from the environment, soil remediation with this technology is not widely explored worldwide17.Soil contaminated with organochlorine pesticides was cleaned using the Fenton oxidation method, which involves Fe0, EDTA, and air. DDT degraded and was removed from contaminated soils efficiently at room temperature, neutral pH, and atmospheric pressure31. DDT and DDE were removed from contaminated soils using soil washing and the photo-Fenton oxidation process. Washing with TritonX-100 solution removes 66% DDT and 80% DDE from soils. After 6 hours of solar Fenton oxidation, 99% of DDT and 95% of DDE were removed from wastewater32.
The total transformation of organic contaminants into CO2 and H2O with zero-waste sludge is one of the benefits of this oxidation technique. Another benefit of this technique is its capacity to degrade a wide variety of pollutants in a single process without being selective5. Acid pH dependency is the key barrier, with an ideal range in an aqueous medium of 2.8 to 3.0. This makes it hostile to soil-based microorganisms and may even change its features, and can hamper agriculture of a large number of important crops17.
Persulfate-based advanced oxidation
AOP becomes a more viable method for degradingdeveloping organic contaminants. The sulfate radical has recently been investigated for its marked superiority and potential for use in the degradation of emerging contaminants due to its elevated oxidation potential (E0 = 2.5-3.1 V), which is similar to •OH (E0 = 2.8 V), as well as it's non-selective to the contaminants. Furthermore, because of their high solubility, these solid persulfate oxidants are easy to transport and apply33. The activation methods for persulfate - including homogeneous catalysis (such as heat, UV, ultrasonic, and alkaline) and heterogeneous catalysis with metal or carbon catalysts - are of utmost importance. Homogeneous catalysis is more typically utilized for soil remediation34. The thermoactivated persulfate oxidation process was used to study chlorpyrifos degradation. Around 30% of chlorpyrifos was degraded at 70 ° C, with a decay rate of (1.8 ± 0.5) × 10-3 min-1. Increasing the temperature could hasten the degradation of persulfate; as a result, the elevation of oxidizingcomponents, especially would increase, facilitating the oxidation of the target compounds35. Using activated persulfate with ferrous and copper ions, propachlor was broken down. Early on, persulfate activation by Fe2+ ions lead toa fast breakdown, but this was soon followed by a sharp decline in efficiency because the sulfate radicals quickly depleted Fe2+.At higher Cu2+ concentrations, however, Cu2+ activated persulfate has a longer decomposition effect and correspondingly higher decomposition enhancement36.According to one study, heat-activated persulfate degrades atrazine effectively. After 2 hours of treatment at 60 °C in the presence of 1 mM persulfate, 50 M atrazine completely disappeared. Increased initial concentrations or temperatures of persulfates significantly improved the efficiency of decomposition37.
When compared to traditional oxidation methods such as H2O2, permanganate (MnO4), O3, and persulfate relatively stronger oxidant persistence and increased redox potential (E0 = 2.01 V) allow it to reach the polluted zone area across long-distances in the subsurface.Moreover, the utilization of persulfate for soil remediation requires lesser soil oxidant demands compared to H2O2 and permanganate, thereby rendering it a more economical approach.In real-world soil remediation with a low soil-to-water ratio, achieving ideal mixing between activator-oxidant and/or oxidant-soil is challenging.As and •OH have a short lifespan and strong oxidation, they may decrease before reaching deeper soil layers. Consequently, the efficiency of degradation can decline with increasing soil thickness34.
TiO2Photocatalysis
Photocatalysis, a method of environmental remediation in which semiconducting metal oxides act as a catalyst, has piqued the interest of researchers in recent decades. Titanium dioxide (TiO2), gallium phosphide (GaP), nickel oxide (NiO), tungsten trioxide (WO3),zinc oxide (ZnO), and cadmium sulfide are semiconductors used as catalysts in photocatalysis studies (CdS). TiO2 stands out as the most effective photocatalyst due to its non-toxic nature, exceptional photoactivity, resistance to chemical reactions, ease of accessibility, and affordability29. According to research, aniline is completely degraded in photocatalysis using only TiO2 or ozone. Photocatalysis techniques have many advantages in aniline degradation, and the photocatalyst can be reused through regeneration, lowering the cost of chemicals used in the studies 38.
TiO2 photocatalysis was employed to degrade diuron in contaminated soil using an ex-situ approach.For up to 120 hours, the experimental setup was exposed to solar light. The top 4 cm of the contaminated soils demonstrates effective diuron destruction by TiO2 photocatalyst39. The maximum degradation of pyridaben was achieved by performing a photocatalysis study with TiO2 and UV light irradiation at 300 nm and 360 nm UV wavelength. The complete removal of pyridabed achieved within 60 min and 140 min for 300 nm and 360 nm respectively40.
Plasma oxidation and ozonation
The technique creates high-voltage electrons, which activate reactive molecules such as ozone (O3), hydroxyl (OH), oxygen (O), and hydrogen peroxide (H2O2) to form free radicals17.One of the most significant active species in plasma discharge processes that contributes to the breakdown of organic pollutants is H2O229.The benefits of plasma oxidation include (i) high efficiency in producing a variety of oxidizing agents and radicals, (ii) the ability to treat various contaminants with varying concentrations, and (iii) the contaminated soils requiringlittle pretreatment. The plasma oxidation reaction, on the other hand, is relatively uncontrollable. The large volume of plasma generation and sustainment is a significant challenge for the widespread application of this technique41.
Ozonation is another oxidation process that is used to degrade contaminants in soil. The application of ozone and ultraviolet radiation generates hydrogen radicals, and these hydrogen radicals oxidize pesticide contaminants in the media. Ozonation has proven to be an effective oxidation method for eliminating pesticide pollutants from both soil and wastewater during the treatment process. By increasing the concentration of ozone, the rate of oxidation can be accelerated. By increasing the ozonation time and pretreatment humidification, the reaction kinetics of ozonation can be improved30.
Electro-kinetic remediation
In this method, two electrodes are inserted into the soil to generate low voltage currents of mA/cm2, causing the movement of pollutants into the soil. This method effectivelyremovespolar biomolecules and heavy metals from soil, sludge, and sediments13.This technology has been used for decades to remediate heavy metals contaminated with heavy metals., and is now being used to remove organic substances. The extraction of organic pollutants typically entails the interplay of electroosmotic water flow and electromigration of ions to the appropriate electrode20.The elimination of pollutants from the electrode’s surface can be achieved by precipitation, ion complexation, or pumping. A significant drawback associated with the use of this procedure has been the potential for the precipitation of elements such as heavy metals near the cathode13.Electro-kinetic (EK) and electro-kinetic Fenton coupled (EKF) technologies were used to remove HCSs and DDT. The elimination efficiency of HCHs (30.5%) and DDT (25.9%) is lower in individual EK. Even though the EKF has a higher degradation rate of 60.9% for HCHs and 40% for DDT42.The removal of 2,4-dichloro phenoxy acetic acid (2,4-D) from contaminated soil was accomplished using electrokinetic soil flushing techniques. For 40 days, a fully automated ex-situ bench-scale setup was run. The results show that 50% of the 2,4-D was removed, 25% remained in the soil, and the remaining 25% was volatilized43.
Electrokinetic remediation technology is rapidly being utilized to remove organic, inorganic, explosives, radionuclides, and other contaminants in contaminated soils and wastewater.
This technique is effective for removing polar pesticides such as organochlorine. In a bench-scale treatment test (140 minutes), 85% of the chlorophenol was removed44. The prime benefit of electrokinetic technique is its low cost and ability to be used both insitu and ex-situ11.
Physical Methods
Thermal Incineration
Thermal incineration is another commonly used remediation technology for organically contaminated soils. Organic contaminants are destroyed at high temperatures with high oxygen content, converting the contaminants to inorganic carbon dioxide and water. Thermal incineration effectively removes pesticides, halogenated and non-halogenated compounds, dioxins, and PCBs from contaminated soil13.This technique can remove contaminants at a rate of 99.99% or higher. High-temperature incinerators have been shown in studies to remove up to 99.9999% of PCBs and dioxins11.
In one study, PCBs contaminated sites were remedied using Infrared High-Temperature Incineration (IHTI) and Base Catalyzed Decomposition (BCD).Both methods resulted in a total amount of environmental damage. The IHTI produced carcinogens, respiratory inorganics, and organics during primary and secondary combustion, which cause terrestrial acidification, eutrophication, and global warming45.
Thermal desorption
Thermal desorption can be utilized to treat contaminated soils containing volatile and semi-volatile toxicants such as PAHs, PCBs, total petroleum hydrocarbon (TPH), and DDT. The primary benefit of this technique over other technologies are that soil and contaminants can be recycled, there is no secondary pollution, it is highly efficient (99%), the treatment period is short, it is safe, and It can handle a variety of contaminants. The contaminants of interest are separated and removed from the soil, either directly or indirectly, by heating in a vacuum or a carrier gas46.Soils contaminated with DDT, DDD, DDE, Toxaphene, and hexachlorohexane were collected from a contaminated site, and thermal desorption was used to remove these contaminants. After 30 minutes at 350oC, the thermal desorption technique removed more than 98% of each pollutant from the collected soils except DDE47.
Thermal desorption is a highly successful technique for eliminating pesticides from contaminated soil. The temperature should be higher than the boiling point of the least volatile contaminants for the removal of a pesticide mixture47.
One of the main disadvantages of thermal desorption is the ex-situ method, which necessitates the excavation and transfer of polluted soil to the treatment location and is costly.
Various toxic gases were formed during thermal desorption, resulting in air pollution6.
Adsorption
Adsorption has been used to remove pesticides from soil and water. The most commonly used adsorbent in this technique is activated carbon. Activated carbon is used to clean up pesticides from pesticide manufacturing plants30. Surfactants can be used to increase the rate of adsorption of inorganic contaminants. These surfactants reduce surface tension, improve solvency, increase micellar solubilization, and aid in pesticide contamination extraction16.Adsorption-activated carbon was used to assess the adsorption and removal potential of diazinon. The adsorption result showed that NH4Cl-induced activated carbon removed 97.5% of 20 mg/L diazinon (NAC)48. Granular activated carbon and pitch-based activated carbon fibers (ACF) were used in an atrazine adsorption study (GAC). The activated carbon fibers absorbed seven times more than the commercially available granular activated carbon. The main reason for this result is the AFC surface area. ACF has a surface area of approximately 1700m2/g and GAC has a surface area of approximately 1100m2/g49.
Various studies have found that organic acids, in the order citrate > oxalate > acetate, improve the adsorption of activated carbon electrodes for contaminant extraction or remediation performance50. OCPs-contaminated soils can be selectively remedied using surfactant-enhanced washing in conjunction with activated carbon21.
Ultrasonic technology
Ultrasound waves are not detectable by the human ear. Ultrasound works primarily by forming cavitation bubbles in the matrix. The chemical reaction in the matrix is accelerated by the implosion of cavitation bubbles, microturbulence, high-speed collisions between matrix particles, and the formation of matrix microporous particles. Due to the continuous formation and collapse of cavitation bubbles, which causesthesonolysis of water to produce free radicals, the local temperature and pressure within the matrix rise dramatically5.The chemical reaction within the matrix is propelled by the intense forces of high-frequency sound waves, reaching up to 18 kHz, as well as high-pressure levels of up to 50 MPa, and scorching temperatures of up to 4726 °C, which degrades the contaminants. Ultrasound was used to remove diazinon in various concentrations. The results showed that increasing diazinon concentration (800, 1200, and 1800 ppm) increased degradation efficiency, but increasing solution volume decreased degradation efficiency51.Chlorpyrifos and Azinphos-methyl pesticides were degraded using an ultrasound method. The results showed that both contaminants degraded quickly using the ultrasound method. Within 20 minutes of being exposed to 130 kHz ultrasound, 98.96% chlorpyrifos and 78.50% azinphos-methyl were degraded52.
This technology can effectively remove different types of toxicants from the soil, including heavy metals, pesticides, hydrocarbons, chlorinated solvents, and petroleum hydrocarbons. The benefits of this ultrasonic technique include low installation and maintenance costs, as well as requiring less energy and space16. The power of the ultrasound, frequency, temperature, intensity, duration of the application, and soil particle size are all factors that influence ultrasonic technique performance53.
Nanotechnology
Nanotechnology can effectively reduce pesticide pollution. Nanoparticles have been used in a variety of biological disciplines, ranging from environmental studies to molecular biology, due to their diverse morphology and size. Iron-based nanoparticles like Fe2O3, Fe3O4, and nano zero-valent iron (nZVI) are commonly utilized in nanocomposites for the efficient removal and detoxification of organic pollutants from soil. These iron nanoparticles oxidize more easily and form pesticide aggregates. Other suitable technologies can easily degrade these pesticide aggregates10.
Using zero-valent iron(Fe0), triazine dechlorination from soil was accomplished. Several studies have shown the mineralization of metolachlor and atrazine can improve remediation5. Iron nanoparticles have shown satisfactory results in removing contaminants in long-term treatment and different soils54. Using Fe nanoparticles, all types of organochlorine pesticides and their metabolites can be removed via photocatalysis and adsorption55. In the presence of light, nanoparticles can act as catalysts, reacting with pesticides to produce harmless molecules such as H2O, N2, and CO256.
Biological Methods
Bioremediation by microorganisms
Biodegradation occurs naturally when soil-dwelling organisms degrade and metabolize various xenobiotic compounds and pesticides for nutrient supply. Soil microbes naturally mineralize various organic and inorganic compounds. These microbes' degradation capability can be increased in a short period with some modifications.
Four factors influence the speed of bioremediation in soil, including the availability of pesticides or their by-products to microorganisms, the physiological condition of those microbes, the survivorship of pesticide-degrading microbes at polluted sites, and the maintenance of a stable population of this microorganisms57.A diverse group of bacteria can degrade different pesticides in soil (Table 1).
Table 1: Pesticide degradation by various bacterial strains.
Sl. No | Pesticide | Bacterial strain | Degradation efficiency |
Organochlorine pesticides | |||
1. | DDT | Bacillus sp. Staphylococcus sp. Stenotrophomonas sp. | The degradation rate ranges from 28.48 to 58.08% when isolates were tested individually, but the rate increased to 82.63% when the mixed culture was used and the incubation period was 31 days58. |
2. | Chlordane | Streptomyces sp. | Following 28 days of incubation 56% of chlordane was removed from soil sample59. |
3. | Lindane | Streptomyces sp. | After 96 h of incubation 46 to 68%, lindane was removed60. |
4. | ?-HCH | Pseudomonas sp. | After 10 days of incubation, 47.3ppb ?-HCHremained in the medium61. |
Organophosphate pesticide | |||
5. | Diazinon | Pseudomonas peli Burkholderiacaryophylli | The degradation rate increases from 3.35 4.26 mg/l/d to 4.55, and 5.36 mg/l/d respectively when supplemented with 0.5% glucose 62. |
6. | Malathion | Acinetobacter johnsonii | The maximum degradation rate was 3.5837 mg/(L·h)63. |
7. | Chlorpyrifos | Bacillus cereus | After 7 days of incubation, 73.9% chlorpyrifos was removed from the medium64. |
Carbamate pesticide | |||
8. | Carbofuran | Flavobacterium sp., Pseudomonas sp., Sphingomonas sp. | Approximately 98% carbofuran in 96 h of incubation 65. |
9. | Carbaryl | Bacillus sp. Morganella sp. | The degradation rates for both isolates were 94.6% and 87.3% respectively, 66. |
Pyrethroid pesticide |
|
| |
10. | Cypermethrin | Bacillus thuringiensis | Approximately 80% of the initial cypermethrin degraded within 15 days of incubation67. |
11. | Cyfluthrin | Photobacterium ganghwense | After 120 h of incubation, 92.13% cyfluthrin was degraded68. |
12. | Deltamethrin | Microbacteriumchocolatum | The deltamethrin degradation rate was 76% in agricultural soils 69. |
Neonicotinoids pesticide |
|
| |
13. | Imidacloprid | Ochrobactrum sp. Rhizobium sp. | After 48 h of incubation approximately 67.67% was degraded70. |
14. | Clothianidin | Pseudomonas stutzeri | Clothianidin degradation was approximately 62% within two weeks71. |
15. | Acetamiprid | Micrococcus luteus | Maximum degradation rate was 69.84% in 24 h72. |
Fungi are another biological agent that is commonly used for pesticide biodegradation and bioremediation (Table 2). Depending on the functional groups in the pesticides, different fungal strains can perform different biodegradation processes such as dechlorination, demethylation, esterification, deoxygenation, oxidation, and dehydrochlorination73.
Table 2: Pesticide degradation by various fungal strains.
Sl. No | Pesticide | Fungal strain | Country &References |
1. | Chlorpyrifos | Acremonium sp. | The degradation rate was highest at 83.9% in a full nutrient medium74. |
2. | ??cypermethrin | Eurotiumcristatum | The half-lives of ??cypermethrinrange from 3.382 to 11.517 days75. |
3. | 3?phenoxybenzoic acid | Eurotiumcristatum | The half-lives of ??cypermethrinrange from 1.749 to 3.194 days75. |
4. | DDT | Phlebiaacanthocystis, Phlebiabrevispora | After 21 days of incubation, both isolates degrade 70 and 30% of DDT respectively76. |
5. | Tribufos, azinphos-methyl, terbufos, and phosmet | Phanerochaetechrysosporium, Pleurotusostreatu,sBjerkanderaadusta | After four days of incubation, these three isolates degrade 50 to 96% of the selected organophosphorus pesticides77. |
7. | Endosulfan | Aspergillus niger | Complete mineralization of 400 mg/ml endosulfan was achieved in 12 days of incubation78. |
8. | Lindane | Aspergillus fumigates | Approximately 94% of lindane was degraded in 72 h of incubation79. |
9. | Lindane or hexachlorocyclohexane | Fusariumpoae and Fusariumsolani | The degradation of lindane by F. poae was 56.7 and 59.4% by F. solani80. |
10. | Monocrotophos | Aspergillus oryzae | In 50 h of incubation, 70% of monocrotophos was degraded and at 168 h the pesticide became undetectable81. |
There are some disadvantages to using fungi as a biodegradative agent, such as the fact that fungal biodegradation is much slower than bacterial biodegradation and that fungal degradation cannot completely remove pesticides73.
Bio-airsparging
The technique of bio-airsparging can be employed to decrease the adsorption of volatile chemicals in the soil, dissolution in underground water, or the saturated zone whenever required. It is a biological technique that involves regularly injecting nutrients and oxygen into the saturated zone to boost the activity of microbes (Figure 4). This indigenous in-situ technology generally employs microorganisms and is less effective in the presence of non-biodegradable contaminants and non-stoppable pollutants13.
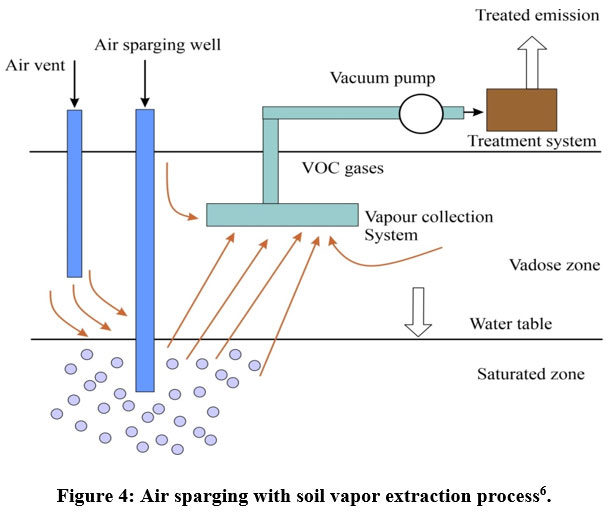 | Figure 4: Air sparging with soil vapor extraction process6.
|
Biosparging has been studied for the removal of toluene, ethylbenzene, benzene, and xylenes (BTEX) from petroleum-hydrocarbon spill sites. Within a 10-month remediation period, more than 70% of BTEX was removed using the biosparging system82. The primary drawback of this method is its slow rate of degradation, which can be time-consuming.
Bioventing
The venting process is critical because air injection occurs in contaminated media, minimizing the off-gassing of volatilized contaminants while maximizing in-situ biodegradation to the atmosphere at a predetermined rate. Bioventing only pumps air into the unsaturated zone, as opposed to bio-sparging, which pumps nutrients and air into the saturated zone6. The method of utilizing an anaerobic gas mixture containing a reducing agent for bio-venting was studied to eliminate DNT and DDT. The results showed that DDT was halved in 8.5 months with the presence of 1,1-dichloro-2,2-bis(p-chlorophenyl) ethane as an intermediate substance. On the other hand, DNT was eliminated within six months without the need for an intermediate compound83. It was possible to access bio-venting studies at two different temperatures. Soils contaminated with toluene and decane were treated at 10oC and 20oC. The results showed that at 20oC, 99.8% and 98.7% reductions in toluene and decane were achieved, respectively. At 10oC, it required 1.6 times the duration and 1.4 times the volume of air to accomplish an equivalent outcome84.
Despite its high level of diversity, the primary principle of this technology is to ensure adequate airflow rates to supply enough oxygen to the contaminated area. This, in turn, facilitates the degradation of organic compounds through soil microorganisms11.
Landfarming
Land farming is the simplest bioremediation technique, requiring little expertise and capital. The introduction of contaminated soils, sediments, or sludges into the top layer of soil followed by periodic aeration enhances the microbial breakdown of the mixture85. Ex-situ and in-situ land farming can be practiced depending on the depth of different polluted zones in the soil. In the practice of remediation, the process of excavating and treating contaminated soil in its original location is referred to as in situ treatment. Excavation for bioremediation is not required if the pollutants are less than 1m beneath the soil surface; however, if the pollutants are more than 1.7m beneath the soil surface, the contaminated soil is excavated to the surface for effective remediation by autochthonous microorganisms16.
Transporting contaminated soil to the land farming site, mixed into the soil surface almost 10 centimeters thick in the case of ex-situ processing. It is a simple technique that requires little infrastructure and is less expensive. Landfarming was used in soil bioremediation where the soil was highly polluted with hexachlorocyclohexane (HCH) isomers (>5 g/kg).The researchers made a notable discovery regarding the four isomers under study. They observed that the ? and ? isomers exhibited a remarkable removal rate of 89% and 82%, respectively. However, the ? and ? isomers displayed a barely noticeable decrease in behavior10.Soils heavily contaminated with hexachlorocyclohexane (HCH) were used for land farming. After 11 months of treatment, 89% ?-HCH and 82% ?-HCH were removed. The metabolites were identified as Pentachlorocyclohexene and tetrachlorocyclohexene86.
Biopiles
Biopiles add a piping system to a pile of contaminated soil, causing the pollutants to decompose aerobically by providing oxygen. To encourage microbial activity, nutrients are administered on the surface of the soil pile (Figure 5). The piles should be 3-4 meters tall and have a volume of tens to hundreds of cubic meters10.
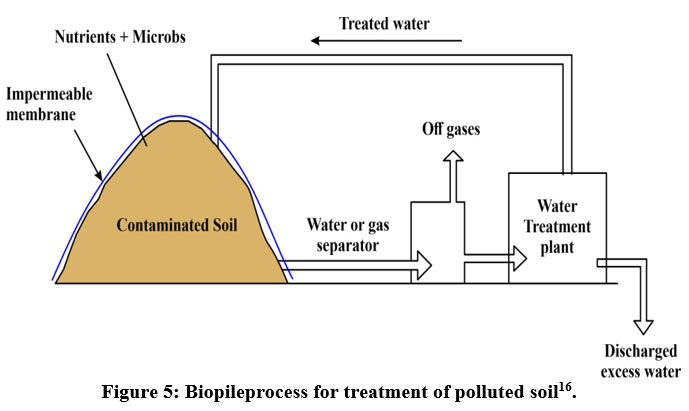 | Figure 5: Biopileprocess for treatment of polluted soil16.
|
The pile configuration boasts a primary benefit, wherein a considerable amount of polluted soil can be remediated within a limited expanse. However, installing and maintaining a pile system is costly. Another disadvantage of this method is that the hot air generated by the pile dries out the soil, reducing soil microbial activity16.
Biopiles were used in a study to remove TPH from contaminated soil. After 66 days of incubation, 85% of TPH wasremoved from the contaminated soil87.
Composting
Another ex-situ solid-phase biological remediation technology is composting. The degradation of organic materials by increased biological activity requires a temperature range of 55 to 65oC for this technique. The blending of polluted earth with natural substances like plant waste and timber chips is undertaken to enhance its texture and oxygenation. To achieve a more effective composting process, one can manipulate environmental elements such as soil acidity, dampness, warmth, nourishment, and carbon-to-nitrogen ratio11.
The composting technique is primarily determined by the bioavailability and bioaccessibility of pollutants to soil microbes, as well as the pesticide composition in the soil10.The composting study was performed in a rotary drum composter for the removal of aldrin, endosulfan ?, endosulfan?,and lindane. In optimal temperature, pH,and moisture the degradation efficiency was 85.67% for aldrin, 84.95% for endosulfan ?, 83.20% for endosulfan ?, and 81.36% for lindane respectively within 7-8 hours88.
Bioslurry
Slurry bioreactors is an ex-situ technique where under controlled environmental conditions recalcitrant pollutants in soil are treated16. To improve contact between soil microorganisms and pollutants, water is mixed with contaminated soil to form a slurry. The slurry is then placed in the bioreactor to control environmental variables. Inoculation can be done regularly to improve degradationbecause all environmental variables in the slurry bioreactor are controlled and optimized, and the degradation rate is much speedier than in other biological technologies. The processed material is appropriate for immediate land application, much like soils that have undergone composting. The maximum time for complete contamination removal using this method is twelve months11.Bio slurry remediation studies for PAH-contaminated soil were conducted. The remediation process could yield a positive outcome due to specific operational conditions, including maintaining a temperature range of 20oC-25oC, doubling the amount of water in relation to the soil, and applying an aeration flux of 60 L/h. After 34 days, Pyrene exhibited a degradation of 90%, whereas benz[a]anthracene experienced a degradation of 33.3%89.
Control over critical environmental parameters, as well as their optimization and monitoring for the bioremediation process, are advantages of this technique over other technologies. One of the main drawbacks of this method is the high cost of installing and managing bioreactors10.
Phytoremediation
Phytoremediation is another innovative technology that is economically and ecologically advantageous4. The primary goal of phytoremediation is to detoxify or extract pollutants from the soil through the use of plants. Through the implementation of three plant-based mechanisms, we can address the issue of environmental pollutants. Firstly, phytoextraction is the absorption and buildup of harmful compounds in the leaves and stems of plants. Secondly, phytodegradation is the enzymatic transformation of these contaminants. Finally, rhizoremediation involves the release of organic acids, sugars, amino acids, and microbial growth factors in the plant root zone to promote the growth of beneficial soil microbes85(Table 3).
Table 3: Plants associated with phytoremediation of pesticides.
Pesticide | Plant species | Remediation |
Dimethoate and malathion | Amaranthus caudate, Lactuca sativa, Nasturtium officinale, Phaseolus vulgaris | Four plant species were used to detoxify the dimethoate and malathion-contaminated soil in the Kingdom of Saudi Arabia. For 50% removal of malathion & dimethoate, Nasturtium officinale takes 25 days, Lactuca sativa takes 23 and 30 days, Amaranthus caudate takes 24 and 28 days, and Phaseolus vulgaris takes 25 and 30 days respectively90. |
Malathion, demeton-S-methyl | Myriophyllum aquaticum, Spirodelaoligorrhiza L., Elodea canadensis | Elodea canadensis, Spirodelaoligorrhiza L., and Myriophyllum aquaticum transform demeton-smethyl and malathion in a similar manner, and after eight days of incubation, the transformation ranges from 83-95 and 29-48% for all three plants91. |
Cypermethrin | Pennisetum pedicellatum | Rhizoremediation of cypermethrin was done by Pennisetum pedicellatum. 65-100% removed from the soil in 60 days. Aerobic, gram-negative bacteria Stenotrophomonas maltophilia is the main degradative agent10. |
Ethion | Eichhornia crassipes | Phytodegradation and plant absorption may be the main mechanisms for ethion elimination by the plant, according to research on the ability of water hyacinth to do so. Ethion accumulated in shoots and roots was reduced by 55-91% and 74-81% respectively92. |
Phytoremediation has many advantages, including a lower cost than other remediation technologies currently available. It also improves soil properties, reduces soil erosion, increases soil microbial diversity, and so on. Aside from these benefits, this technology has some drawbacks, including climatic conditions, plant tolerance to contaminants, a longer remediation duration for the restoration of contaminated land, and the concentration and bioavailability of the pollutants. This technique is only appropriate for sites with low contaminant concentrations that are dispersed over a large area10.
Discussion
Regarding immobilization, there are techniques such as pumping, draining, capping, clay slurry wall, solidification, and vitrification that prevent the movement of contaminants. Regarding organics and pesticides, solidification has poor efficacy and in the case of vitrification soil depth, long-term monitoring, and non-movable organics are the main limiting factor. However, an additional technique is necessary for the complete removal of the pollutants. Separation technology involves washing and soil flushing to remove contaminants, but this method requires an additional technique to remediate the contaminants completely, which is more expensive and can pose risks to the environment due to the use of synthetic surfactants. The problem with solvent extract is the production of toxic contaminants that needs further advanced treatment. Cyclodextrin can be more effective than other separation techniques because requires less time to mitigate bulk material at a low price. Oxidation processes involve the use of iron ions, light, ultrasonic waves, semiconducting metal oxides, plasma, ozone, ultraviolet radiation, and persulfate to oxidize organic pollutants in the soil. This method works well for totally mineralizing pollutants.The oxidation rate is also affected by the presence of dissolved oxygen, dissolved solids,competitive substrates, and so on.Although chemical treatments are capable of effectively treating contaminants present in high concentrations and have faster results, they can be more costly and harmful to the soil due to the use of intense heat, potent acids, and alkaline substances.
In the electro-kinetic process, a low voltage current is used to move heavy metals and organic-inorganic pollutants towards the electrode. Thermal incineration is an efficient technique for converting pollutants into CO2 and water, but it produces carcinogens and respiratory inorganics and causes acidification, eutrophication, and global warming. It is also an ex-situ process, making it more expensive.Thermal desorption involves high temperatures and soil excavation and the efficiency of desorption can be more than 99%, which produces harmful gases. Pollution transfer from one medium (soil) to another is one of the main drawbacks of thermal treatments (gas). If the gases generated from thermal desorption are taken care of, then this is a fairly environmentally friendly technology. Adsorption with activated carbon and surfactants is useful for removing pesticides, but it has limitations in field applications. Sono-lysis requires high frequency, pressure, and temperature, making it an ex-situ process that depends on soil type and pesticide properties. The use of iron-based nanoparticles can effectively reduce pesticides from soil and can be enhanced by photocatalysis. Physical treatment necessitates some equipment installation as well as ex-situ treatments by excavating the contaminated soil to the treatment site. As a result, physical treatments are impractical and cost more than other available technologies
Remediation with micro-organisms is a natural process in which soil microbes break down pollutants into less harmful substances. The rate of degradation can be improved by injecting nutrients and oxygen into the soil to increase microbial growth and activity. Bio-air sparging or soil vapor extraction is an environment-friendly technique that can treat a large amount of soil at a low cost. Effectiveness in reducing volatile organic compounds and low time constrain it became more suitable for in-situ bioremediation technique. Landfarming has some limitations requiring a large amount of land and the contaminants can be transferred to an undisturbed site or atmosphere. Biopiles can be effective for soil with a low concentration of contaminants, the high heavy metal concentration may limit the growth of microbes. Composting and slurry bioreactorsrequire excavation of the contaminated soil to the treatment siteand also require organic additives and can be expensive. Phytoremediation involves using plants to extract or remove contaminants from the soil, which becomes more efficient when combined with soil microbes, especially in rhizoremediation. The main reason for selecting phytoremediation as a bioremediation technique is the minimal environmental disturbance, used on a large range of contaminants, minimal secondary byproducts, and its cost-effectiveness. When addressing organic pollutants, it is essential to prioritize the utilization of plants that facilitate phytodegradation instead of phytoextraction and accumulation. This distinction holds particular significance due to concerns regarding the potential transfer of pollution during crop disposal, as well as the risks associated with pollutant accumulation in the food chain. While biological technologies offer notable advantages, such as their ability to break down pollutants, they do have a few drawbacks. These include longer processing times and the potential for certain pesticides to break down into more harmful by-products during the bioremediation process. Moreover, the use of enriched microbes in contaminated soil can present challenges due to environmental fluctuations in factors such as pH, temperature, nutrient levels, moisture content, and competition from other microbes.
Conclusion
The scientific community is actively addressing the environmental concern posed by pesticides in soils, reflecting their significant attention to this matter. This article offers a comprehensive survey of diverse technologies used to remediate pesticide-contaminated soils. It covers the underlying principles, advantages, disadvantages, advancements, and limitations associated with these technologies. Additionally, the article presents research perspectives for each technology and examines their applicability restrictions.
Chemical treatments for pesticide-contaminated soils are quicker when compared to biological treatments. However,a drawback is that the residues generated from the separation techniques used in chemical treatments require extra treatment or disposal, leading to an escalation in project expenses. Probably the most efficient separationtechnique is the use of cyclodextrins as a pretreatment of the contaminated soil. Chemical treatments can be detrimental to soil microbes and other soil properties, thus limiting the potential future use of these soils. Physical methods are applied to specific areas where contamination of soil with banned pesticides or concentration is high. Adsorption with activated charcoal or thermal desorption is the best technique to mitigate this type of soil contamination problem. A widely used technique in biological remediation is the utilization of fungi and bacteria strains that possess the ability to break down pesticides. Despite its cost-effectiveness and efficiency, the success of this process relies on various factors, including soil nutrient availability, moisture content, oxygen, temperature, and pHlevel.Despite their limitations, composting and landfarming are the most feasible biological methods due to their low implementation costs and immediate readiness for use. Bio-air sparging and phytoremediation can be more applicable for an environment-friendly approach to the remediation of contaminants.
It is crucial to note that there is a dearth of research on the remediation of agricultural soils contaminated by pesticides on a field scale. Thus, further evaluation of the proposed remediation methods' effectiveness and associated costs in real-world field scenarios is necessary.To conclude, it is crucial to consider all the factors involved, such as pH, matrix type, temperature, the quantity of water and soil, investment cost, pesticide solubility, and more, when selecting the most appropriate method and material for pesticide removal. Hopefully, the literature review and discussion section can aid analysts in conducting an initial screening of the best technique based on their requirements.
Acknowledgement
Authors are grateful to Principal, PG Department of Zoology, Raja Narendra Lal Khan Women’s College (Autonomous) Natural and Applied Science Research Centre, Paschim Medinipur, West Bengal, India for the facilities provided and acknowledge the support to PS to carry out the paper as a part of the thesis under Vidyasagar University, Raja Narendra Lal Khan Women’s College (Autonomous). PS also acknowledges CSIR fellowship to carry out the research work.PPC and HKJ acknowledge to DST-CURIE for infrastructural support to Natural and Applied Sciences Research centre, Raja N. L. Khan Women’s College, Paschim Medinipur, West Bengal, India.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Sources
This research work has been supported by CSIR JRF & SRF. File No. 08/745(0001)/2020-EMR-I
References
- Verma JP, Jaiswal DK, Sagar R. Pesticide relevance and their microbial degradation: a-state-of-art. Reviews in Environmental Science and Bio/Technology. 2014;13(4):429-466. doi:10.1007/s11157-014-9341-7
- Sharma A, Kumar V, Shahzad B, et al. Worldwide pesticide usage and its impacts on the ecosystem. SN Applied Sciences. 2019;1(11). doi:10.1007/s42452-019-1485-1
- Satish GP, Ashokrao DM, Arun SK. Microbial degradation of pesticide: A review. African Journal of Microbiology Research. 2017;11(24):992-1012. doi:10.5897/ajmr2016.8402
- Rajmohan KS, Chandrasekaran R, Varjani S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian Journal of Microbiology. 2020;60(2):125-138. doi:10.1007/s12088-019-00841-x
- Marican A, Durán-Lara EF. A review on pesticide removal through different processes. Environmental Science and Pollution Research. 2017;25(3):2051-2064. doi:10.1007/s11356-017-0796-2
- Khan FI, Husain T, Hejazi R. An overview and analysis of site remediation technologies. Journal of Environmental Management. 2004;71(2):95-122. doi:10.1016/j.jenvman.2004.02.003
- Meegoda JN, Ezeldin AS, Fang HY, Inyang HI. Waste Immobilization Technologies. Practice Periodical Hazardous, Toxic, and Radioactive Waste Management. 2003;7(1):46-58. doi:10.1061/(asce)1090-025x(2003)7:1(46)
- Evans J, Ryan C. Time-Dependent Strength Behavior of Soil-Bentonite Slurry Wall Backfill. Waste Containment and Remediation. Published online October 9, 2005. doi:10.1061/40789(168)45
- Rodríguez-Cruz MS, Sánchez-Martín MJ, Andrades MS, Sánchez-Camazano M. Modification of clay barriers with a cationic surfactant to improve the retention of pesticides in soils. Journal of Hazardous Materials. 2007;139(2):363-372. doi:10.1016/j.jhazmat.2006.06.042
- Morillo E, Villaverde J. Advanced technologies for the remediation of pesticide-contaminated soils. Science of The Total Environment. 2017;586:576-597. doi:10.1016/j.scitotenv.2017.02.020
- Lodolo A, Gonzalez-Valencia E, Miertus S. Overview of Remediation Technologies for Persistent Toxic Substances. Arhiv za higijenuradaitoksikologiju. 2001;52(2):253-280. Accessed January 21, 2023. https://hrcak.srce.hr/539
- Fuchun B, Guoxu W, Lihong Z. Study on Stabilization of 36 Insecticides and 33 Pesticide Mixtures. en.cnki.com.cn. Published 2010. Accessed January 21, 2023. http://en.cnki.com.cn/Article_en/CJFDTOTAL-NYKG201005015.htm
- Castelo-Grande T, Augusto PA, Monteiro P, Estevez AM, Barbosa D. Remediation of soils contaminated with pesticides: a review. International Journal of Environmental Analytical Chemistry. 2010;90(3-6):438-467. doi:10.1080/03067310903374152
- Timmerman CL, Zintak LN. Application of In-Situ Vitrification at the Parsons Chemical Site. Remediation Journal. 1998;8(2):75-85. doi:10.1002/rem.3440080208
- Koustas RN, Fischer D. Review of Separation Technologies for Treating Pesticide-Contaminated Soil. Journal of the Air & Waste Management Association. 1998;48(5):434-440. doi:10.1080/10962247.1997.11877501
- Koul B, Taak P. Biotechnological Strategies for Effective Remediation of Polluted Soils. Springer Singapore; 2018. doi:10.1007/978-981-13-2420-8
- Baldissarelli DP, Vargas GDLP, Korf EP, Galon L, Kaufmann C, Santos JB. Remediation of Soils Contaminated by Pesticides Using Physicochemical Processes: a Brief Review. Planta Daninha. 2019;37(0):1-13. doi:10.1590/s0100-83582019370100054
- Islam MN, Jo YT, Jung SK, Park JH. Evaluation of Subcritical Water Extraction Process for Remediation of Pesticide-Contaminated Soil. Water, Air, & Soil Pollution. 2013;224(8). doi:10.1007/s11270-013-1652-8
- Sahle-Demessie E, Meckes MC, Richardson TL. Remediating pesticide contaminated soils using solvent extraction. Environmental Progress. 1996;15(4):293-300. doi:10.1002/ep.670150420
- Zhu L, Lu L, Zhang D. Mitigation and remediation technologies for organic contaminated soils. Frontiers of Environmental Science & Engineering in China. 2010;4(4):373-386. doi:10.1007/s11783-010-0253-7
- Zhang S, HE Y, WU L, et al. Remediation of Organochlorine Pesticide-Contaminated Soils by Surfactant-Enhanced Washing Combined with Activated Carbon Selective Adsorption. Pedosphere. 2019;29(3):400-408. doi:10.1016/s1002-0160(17)60328-x
- Bandala ER, Aguilar F, Torres LG. Surfactant-enhanced soil washing for the remediation of sites contaminated with pesticides. Land Contamination & Reclamation. 2010;18(2):151-159. doi:10.2462/09670513.991
- Von Lau E, Gan S, Ng HK, Poh PE. Extraction agents for the removal of polycyclic aromatic hydrocarbons (PAHs) from soil in soil washing technologies. Environmental Pollution. 2014;184:640-649. doi:10.1016/j.envpol.2013.09.010
- Ye M, Sun M, Liu Z, et al. Evaluation of enhanced soil washing process and phytoremediation with maize oil, carboxymethyl-?-cyclodextrin, and vetiver grass for the recovery of organochlorine pesticides and heavy metals from a pesticide factory site. Journal of Environmental Management. 2014;141:161-168. doi:10.1016/j.jenvman.2014.03.025
- Ye M, Sun M, Hu F, et al. Remediation of organochlorine pesticides (OCPs) contaminated site by successive methyl-?-cyclodextrin (MCD) and sunflower oil enhanced soil washing – Portulaca oleracea L. cultivation. Chemosphere. 2014;105:119-125. doi:10.1016/j.chemosphere.2013.12.058
- Navarro A, Martínez F. The use of soil-flushing to remediate metal contamination in a smelting slag dumping area: Column and pilot-scale experiments. Engineering Geology. 2010;115(1-2):16-27. doi:10.1016/j.enggeo.2010.07.001
- Di Palma L. Experimental assessment of a process for the remediation of organophosphorous pesticides contaminated soils through in situ soil flushing and hydrolysis. Water, Air, and Soil Pollution. 2003;143(1/4):301-314. doi:10.1023/a:1022890529765
- Xueli X, Xinhua Z, Lixiang Z. Effectiveness comparison of different solvents in organochlorine pesticide contaminated soil flushing. Chinese Journal of Environmental Engineering. 2012;6(1):347-352. Accessed January 21, 2023. http://www.cjee.ac.cn/en/article/id/20120166
- Cheng M, Zeng G, Huang D, et al. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chemical Engineering Journal. 2016;284:582-598. doi:10.1016/j.cej.2015.09.001
- Ajiboye TO, Kuvarega AT, Onwudiwe DC. Recent Strategies for Environmental Remediation of Organochlorine Pesticides. Applied Sciences. 2020;10(18):6286. doi:10.3390/app10186286
- Cao M, Wang L, Wang L, Chen J, Lu X. Remediation of DDTs contaminated soil in a novel Fenton-like system with zero-valent iron. Chemosphere. 2013;90(8):2303-2308. doi:10.1016/j.chemosphere.2012.09.098
- Villa RD, Trovó AG, Nogueira RFP. Soil remediation using a coupled process: soil washing with surfactant followed by photo-Fenton oxidation. Journal of Hazardous Materials. 2010;174(1-3):770-775. doi:10.1016/j.jhazmat.2009.09.118
- Song W, Li J, Wang Z, Zhang X. A mini review of activated methods to persulfate-based advanced oxidation process. Water Science and Technology. 2018;79(3):573-579. doi:10.2166/wcc.2018.168
- Jiang C, Yang Y, Zhang L, et al. Degradation of Atrazine, Simazine and Ametryn in an arable soil using thermal-activated persulfate oxidation process: Optimization, kinetics, and degradation pathway. Journal of Hazardous Materials. 2020;400:123201. doi:10.1016/j.jhazmat.2020.123201
- Zhou L, Zhang Y, Ying R, et al. Thermoactivated persulfate oxidation of pesticide chlorpyrifos in aquatic system: kinetic and mechanistic investigations. Environmental Science and Pollution Research. 2017;24(12):11549-11558. doi:10.1007/s11356-017-8672-7
- Liu CS, Shih K, Sun CX, Wang F. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Science of The Total Environment. 2012;416:507-512. doi:10.1016/j.scitotenv.2011.12.004
- Ji Y, Dong C, Kong D, Lu J, Zhou Q. Heat-activated persulfate oxidation of atrazine: Implications for remediation of groundwater contaminated by herbicides. Chemical Engineering Journal. 2015;263:45-54. doi:10.1016/j.cej.2014.10.097
- Bhat AP, Gogate PR. Degradation of nitrogen-containing hazardous compounds using advanced oxidation processes: A review on aliphatic and aromatic amines, dyes, and pesticides. Journal of Hazardous Materials. 2021;403:123657. doi:10.1016/j.jhazmat.2020.123657
- Higarashi MM, Jardim WF. Remediation of pesticide contaminated soil using TiO2 mediated by solar light. Catalysis Today. 2002;76(2-4):201-207. doi:10.1016/s0920-5861(02)00219-5
- Zhu X, Yuan C, Bao Y, Yang J, Wu Y. Photocatalytic degradation of pesticide pyridaben on TiO2 particles. Journal of Molecular Catalysis A: Chemical. 2005;229(1-2):95-105. doi:10.1016/j.molcata.2004.11.010
- Zhang H, Ma D, Qiu R, Tang Y, Du C. Non-thermal plasma technology for organic contaminated soil remediation: A review. Chemical Engineering Journal. 2017;313:157-170. doi:10.1016/j.cej.2016.12.067
- Ni M, Tian S, Huang Q, Yang Y. Electrokinetic-Fenton remediation of organochlorine pesticides from historically polluted soil. Environmental Science and Pollution Research. 2018;25(12):12159-12168. doi:10.1007/s11356-018-1479-3
- Risco C, López-Vizcaíno R, Sáez C, et al. Remediation of soils polluted with 2,4-D by electrokinetic soil flushing with facing rows of electrodes: A case study in a pilot plant. Chemical Engineering Journal. 2016;285:128-136. doi:10.1016/j.cej.2015.10.011
- Gomes HI, Dias-Ferreira C, Ribeiro AB. Electrokinetic remediation of organochlorines in soil: Enhancement techniques and integration with other remediation technologies. Chemosphere. 2012;87(10):1077-1090. doi:10.1016/j.chemosphere.2012.02.037
- Hu X, Zhu J, Ding Q. Environmental life-cycle comparisons of two polychlorinated biphenyl remediation technologies: Incineration and base catalyzed decomposition. Journal of Hazardous Materials. 2011;191(1-3):258-268. doi:10.1016/j.jhazmat.2011.04.073
- Zhao C, Dong Y, Feng Y, Li Y, Dong Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere. 2019;221:841-855. doi:10.1016/j.chemosphere.2019.01.079
- Sahle-Demessie E, Richardson T. Cleaning Up Pesticide Contaminated Soils: Comparing Effectiveness of Supercritical Fluid Extraction with Solvent Extraction and Low Temperature Thermal Desorption. Environmental Technology. 2000;21(4):447-456. doi:10.1080/09593332108618106
- Moussavi G, Hosseini H, Alahabadi A. The investigation of diazinon pesticide removal from contaminated water by adsorption onto NH4Cl-induced activated carbon. Chemical Engineering Journal. 2013;214:172-179. doi:10.1016/j.cej.2012.10.034
- Mart??n-GullónI, Font R. Dynamic pesticide removal with activated carbon fibers. Water Research. 2001;35(2):516-520. doi:10.1016/s0043-1354(00)00262-1
- Yang X, Liu L, Tan W, Liu C, Dang Z, Qiu G. Remediation of heavy metal contaminated soils by organic acid extraction and electrochemical adsorption. Environmental Pollution. 2020;264:114745. doi:10.1016/j.envpol.2020.114745
- Matouq MA, Al-Anber ZA, Tagawa T, Aljbour S, Al-Shannag M. Degradation of dissolved diazinon pesticide in water using the high frequency of ultrasound wave. Ultrasonics Sonochemistry. 2008;15(5):869-874. doi:10.1016/j.ultsonch.2007.10.012
- Agarwal S, Tyagi I, Gupta VK, et al. Degradation of azinphos-methyl and chlorpyrifos from aqueous solutions by ultrasound treatment. Journal of Molecular Liquids. 2016;221:1237-1242. doi:10.1016/j.molliq.2016.04.076
- Effendi AJ, Wulandari M, Setiadi T. Ultrasonic application in contaminated soil remediation. Current Opinion in Environmental Science & Health. 2019;12:66-71. doi:10.1016/j.coesh.2019.09.009
- Bhalerao TS. A review: applications of iron nanomaterials in bioremediation and in detection of pesticide contamination. International Journal of Nanoparticles. 2014;7(1):73. doi:10.1504/ijnp.2014.062034
- Rani M, Shanker U, Jassal V. Recent strategies for removal and degradation of persistent & toxic organochlorine pesticides using nanoparticles: A review. Journal of Environmental Management. 2017;190:208-222. doi:10.1016/j.jenvman.2016.12.068
- Bakshi M, Abhilash PC. Nanotechnology for soil remediation: Revitalizing the tarnished resource. Nano-Materials as Photocatalysts for Degradation of Environmental Pollutants. Published online 2020:345-370. doi:10.1016/b978-0-12-818598-8.00017-1
- Singh DK. Biodegradation and bioremediation of pesticide in soil: concept, method and recent developments. Indian Journal of Microbiology. 2008;48(1):35-40. doi:10.1007/s12088-008-0004-7
- Mwangi K, Boga HI, Muigai AW, Kiiyukia C, Tsanuo MK. Degradation of dichlorodiphenyltrichloroethane (DDT) by bacterial isolates from cultivated and uncultivated soil. African Journal of Microbiology Research. 2010;4(3):185-196. Accessed January 21, 2023. https://elibrary.pu.ac.ke/handle/123456789/556
- Cuozzo SA, Fuentes MS, Bourguignon N, Benimeli CS, Amoroso MJ. Chlordane biodegradation under aerobic conditions by indigenous Streptomyces strains. International Biodeterioration & Biodegradation. 2012;66(1):19-24. doi:10.1016/j.ibiod.2011.09.011
- Fuentes MS, Sáez JM, Benimeli CS, Amoroso MJ. Lindane Biodegradation by Defined Consortia of Indigenous Streptomyces Strains. Water, Air, & Soil Pollution. 2011;222(1-4):217-231. doi:10.1007/s11270-011-0818-5
- Nawab A. Determination of organochlorine pesticides in agricultural soil with special reference to ?-HCH degradation by Pseudomonas strains. Bioresource Technology. 2003;88(1):41-46. doi:10.1016/s0960-8524(02)00263-8
- Mahiudddin M, Fakhruddin ANM, Abdullah-Al-Mahin, Chowdhury M a. Z, Rahman MA, Alam MK. Degradation of the Organophosphorus Insecticide Diazinon by Soil Bacterial Isolate. The International Journal of Biotechnology. 2014;3(1):12-23. Accessed January 21, 2023. https://archive.conscientiabeam.com/index.php/57/article/view/1513
- Xie S, Liu J, Li L, Qiao C. Biodegradation of malathion by Acinetobacter johnsonii MA19 and optimization of cometabolism substrates. Journal of Environmental Sciences. 2009;21(1):76-82. doi:10.1016/s1001-0742(09)60014-0
- Liu ZY, Chen X, Shi Y, Su ZC. Bacterial Degradation of Chlorpyrifos by Bacillus cereus. Advanced Materials Research. 2011;356-360:676-680. doi:10.4028/www.scientific.net/amr.356-360.676
- Mishra S, Zhang W, Lin Z, et al. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere. 2020;259:127419. doi:10.1016/j.chemosphere.2020.127419
- Hamada M, Matar A, Bashir A. Carbaryl degradation by bacterial isolates from a soil ecosystem of the Gaza Strip. Brazilian Journal of Microbiology. 2015;46(4):1087-1091. doi:10.1590/s1517-838246420150177
- Bhatt P, Huang Y, Zhang W, Sharma A, Chen S. Enhanced Cypermethrin Degradation Kinetics and Metabolic Pathway in Bacillus thuringiensis Strain SG4. Microorganisms. 2020;8(2):223. doi:10.3390/microorganisms8020223
- Jayasree VS, Sobhana KS, Poulose P, et al. Biodegradation of the pyrethroid pesticide cyfluthrin by the halophilic bacterium Photobacterium ganghwense isolated from coral reef ecosystem. Indian Journal of Fisheries. 2020;67(4). doi:10.21077/ijf.2020.67.4.100553-14
- Erguven GÖ, Koçak E. Determining The Detoxification Potential of Some Soil Bacteria and Plants on Bioremediation of Deltamethrin, Fenvalerate and Permethrin Pesticides. Eurasian Journal of Agricultural Research. 2019;3(1):36-47. Accessed January 21, 2023. https://dergipark.org.tr/en/pub/ejar/issue/48478/588681
- Hu G. Isolation of an Indigenous Imidacloprid-Degrading Bacterium and Imidacloprid Bioremediation Under Simulated In Situ and Ex Situ Conditions. Journal of Microbiology and Biotechnology. 2013;23(11):1617-1626. doi:10.4014/jmb.1305.05048
- Parte SG, Kharat AS. Aerobic Degradation of Clothianidin to 2-Chloro-methyl Thiazole and Methyl 3-(Thiazole-yl) Methyl Guanidine Produced by Pseudomonas stutzerismk. Journal of Environmental and Public Health. 2019;2019:1-12. doi:10.1155/2019/4807913
- Kanjilal T, Bhattacharjee C, Datta S. Bio-degradation of acetamiprid from wetland wastewater using indigenous Micrococcus luteus strain SC 1204: Optimization, evaluation of kinetic parameter and toxicity. Journal of Water Process Engineering. 2015;6:21-31. doi:10.1016/j.jwpe.2015.03.002
- Maqbool Z, Hussain S, Imran M, et al. Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: a critical review. Environmental Science and Pollution Research. 2016;23(17):16904-16925. doi:10.1007/s11356-016-7003-8
- Kulshrestha G, Kumari A. Fungal degradation of chlorpyrifos by Acremonium sp. strain (GFRC-1) isolated from a laboratory-enriched red agricultural soil. Biology and Fertility of Soils. 2010;47(2):219-225. doi:10.1007/s00374-010-0505-5
- Hu K, Deng W, Zhu Y, et al. Simultaneous degradation of ??cypermethrin and 3?phenoxybenzoic acid byEurotiumcristatum ET1, a novel “golden flower fungus” strain isolated from Fu Brick Tea. MicrobiologyOpen. 2018;8(7). doi:10.1002/mbo3.776
- Xiao P, Mori T, Kamei I, Kondo R. A novel metabolic pathway for biodegradation of DDT by the white rot fungi, Phlebialindtneri and Phlebiabrevispora. Biodegradation. 2010;22(5):859-867. doi:10.1007/s10532-010-9443-z
- Jauregui J, Valderrama B, Albores A, Vazquez-Duhalt R. Microsomal transformation of organophosphorus pesticides by white rot fungi. Biodegradation. 2003;14(6):397-406. doi:10.1023/a:1027316610450
- Bhalerao TS, Puranik PR. Biodegradation of organochlorine pesticide, endosulfan, by a fungal soil isolate, Aspergillus niger. International Biodeterioration & Biodegradation. 2007;59(4):315-321. doi:10.1016/j.ibiod.2006.09.002
- Dey P, Malik A, Mishra A, Singh DK, von Bergen M, Jehmlich N. Mechanistic insight to mycoremediation potential of a metal resistant fungal strain for removal of hazardous metals from multimetal pesticide matrix. Environmental Pollution (Barking, Essex: 1987). 2020;262:114255. doi:10.1016/j.envpol.2020.114255
- Sagar V, Singh DP. Biodegradation of lindane pesticide by non white- rots soil fungus Fusarium sp. World Journal of Microbiology and Biotechnology. 2011;27(8):1747-1754. doi:10.1007/s11274-010-0628-8
- Bhalerao TS, Puranik PR. Microbial degradation of monocrotophos by Aspergillus oryzae. International Biodeterioration & Biodegradation. 2009;63(4):503-508. doi:10.1016/j.ibiod.2008.11.011
- Kao CM, Chen CY, Chen SC, Chien HY, Chen YL. Application of in situ biosparging to remediate a petroleum-hydrocarbon spill site: Field and microbial evaluation. Chemosphere. 2008;70(8):1492-1499. doi:10.1016/j.chemosphere.2007.08.029
- Shah JK, Sayles GD, Suidan MT, Mihopoulos P, Kaskassian S. Anaerobic bioventing of unsaturated zone contaminated with DDT and DNT. Water Science and Technology. 2001;43(2):35-42. doi:10.2166/wst.2001.0070
- Malina G, Grotenhuis JTC, Rulkens WH. The Effect of Temperature on the Bioventing of Soil Contaminated with Toluene and Decane. Journal of Soil Contamination. 1999;8(4):455-480. doi:10.1080/10588339991339414
- Gomes HI, Dias-Ferreira C, Ribeiro AB. Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Science of The Total Environment. 2013;445-446:237-260. doi:10.1016/j.scitotenv.2012.11.098
- Rubinos DA, Villasuso R, Muniategui S, Barral MT, Díaz-Fierros F. Using the Landfarming Technique to Remediate Soils Contaminated with Hexachlorocyclohexane Isomers. Water, Air, and Soil Pollution. 2006;181(1-4):385-399. doi:10.1007/s11270-006-9309-5
- Iturbe R, Flores C, Chavez C, Bautista G, Torres LG. Remediation of contaminated soil using soil washing and biopile methodologies at a field level. Journal of Soils and Sediments. 2004;4(2):115-122. doi:10.1007/bf02991055
- Ali M, Kazmi AA, Ahmed N. Study on effects of temperature, moisture and pH in degradation and degradation kinetics of aldrin, endosulfan, lindane pesticides during full-scale continuous rotary drum composting. Chemosphere. 2014;102:68-75. doi:10.1016/j.chemosphere.2013.12.022
- Gong Z, Li P, Guo S, Jing X, Wang X, Zhang H. Bioslurry remediation of soil contaminated with polycyclic aromatic hydrocarbons. Huan jingkexue= Huanjingkexue. 2001;22(5):112-116. Accessed January 21, 2023. https://europepmc.org/article/med/11769215
- Al-Qurainy F, Abdel-Megeed A. Phytoremediation and Detoxification of Two Organophosphorous Pesticides Residues in Riyadh Area. World Applied Sciences Journal. 2009;6(7):987-998.
- Gao J, Garrison AW, Hoehamer C, Mazur CS, Wolfe NL. Uptake and Phytotransformation of Organophosphorus Pesticides by Axenically Cultivated Aquatic Plants. Journal of Agricultural and Food Chemistry. 2000;48(12):6114-6120. doi:10.1021/jf9904968
- Xia H, Ma X. Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresource Technology. 2006;97(8):1050-1054. doi:10.1016/j.biortech.2005.04.039






