Environmental acceptable synthesis of 2-[(5/-(substituted phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Meghasham Narule1 and Jyotsna Meshram1 *
1
Department of Chemistry,
RTM Nagpur University,
Nagpur,
440 033
India
DOI: http://dx.doi.org/10.12944/CWE.1.1.08
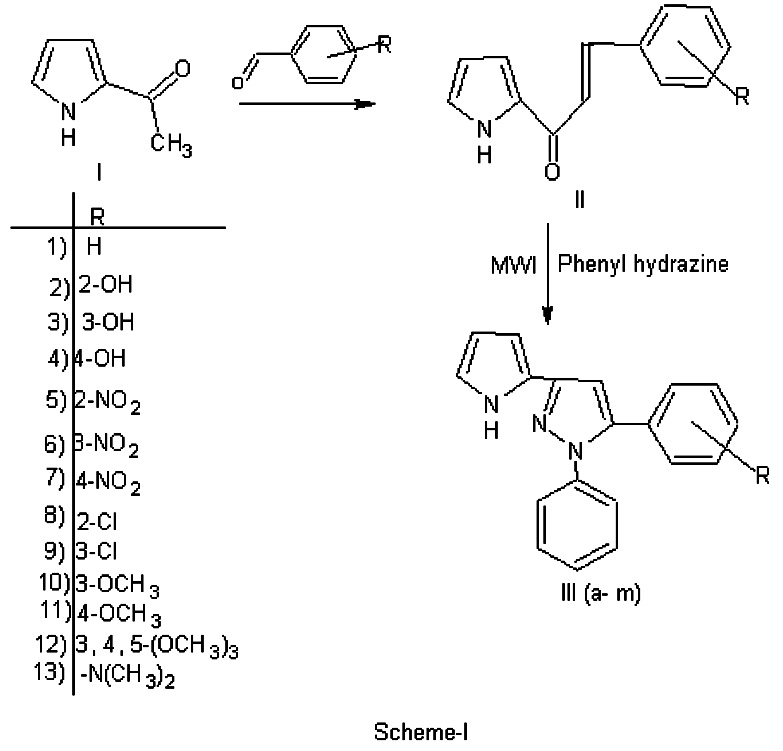
An efficient synthesis of 2-[(5/-(substituted phenyl)-1/ -phenyl) pyrazolyl] pyrroles III (a-m) starting from 2-acetyl pyrrole I and 2-[substituted benz-1/(propene-1//-one)] Pyrroles II (a-m), in the presence of phenyl hydrazine, 2-[(5/-(4//-substituted phenyl)-1/ -phenyl) pyrazolyl] pyrroles III (a-m) has been synthesized in microwave oven for the first time. On comparing with the classical methods, this method has advantages such as shorter reaction time, better yield, simple workup and environmental acceptability.
Copy the following to cite this article:
Narule M, Meshram J. Environmental acceptable synthesis of 2-[(5/-(substituted phenyl)-1/ -phenyl) pyrazolyl] pyrroles. Curr World Environ 2006;1(1):45-50 DOI:http://dx.doi.org/10.12944/CWE.1.1.08
Copy the following to cite this URL:
Narule M, Meshram J. Environmental acceptable synthesis of 2-[(5/-(substituted phenyl)-1/ -phenyl) pyrazolyl] pyrroles. Curr World Environ 2006;1(1):45-50. Available from: http://www.cwejournal.org/?p=515
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2006-03-25 |
|---|---|
| Accepted: | 2006-05-21 |
Introduction
In recent years the pollution free organic reaction and assisted by microwaves in particular have been gained special attention.1,2 The use of solid supports as well as microwaves3 has been well established as a pollution free technique, which is currently under much investigation by synthetic organic chemists.4 As Microwave induced Organic Reaction Enhancement (MORE) chemistry5,6 allows reaction to occur on a preparative scale in open vessels under solvent free conditions which avoids the risk of high pressures and explosions7,8 microwave activation rather than conventional heating is preferred as solid supports are rather poor thermal conductors but strong microwave absorbents.9 One reaction is that the use of microwave activation in organic synthesis can increase the purity of the resulting products, enhance the chemical yield and shorten the reaction time. The other reason is that solvent free reaction avoids organic solvents during the reaction in organic synthesis, lead to a clean, efficient and economical technology.10 It has many advantages such as high efficiency and selectivity, easy separation and purification, mild reaction conditions and environmental acceptability,11 further the reactions are generally faster and the products obtained are of high purity,12 for increase the efficiency of microwave reaction number of inorganic solid supports like, alumina, silica gel, montmorillonite K10 clay, etc are used as catalyst and as energy transfer, medium that devoid the hazards of solution phase reactions. These solid sup portal microwave organic reaction13 show various uses such as better selectivity, remarkable reaction rate enhancement, improved yield and associated ease of manipulation.14,16
Nitrogen containing heterocyclic compounds17 like pyrroles and pyrazoles have received considerable attention in recent years due to their biological activities like anti-inflammatory, anlagen, anticonvulsant and antidiabetic.18
Results and Discussion
In view of these observations, it was thought worthwhile to synthesize and investigate the compounds in which pyrazole have been linked with pyrrole moiety.
The reaction sequence leading to the formation of desired heterocyclic compounds are outlined in Scheme-I synthesis of 2-[(5/-(4//-substituted phenyl)-1/ -phenyl) pyrazolyl] pyrroles III (a-m) starting from 2-acetyl pyrrole I and 2- [substituted benz-1/(propene-1//-one)] Pyrroles II (a-m), in the presence of phenyl hydrazine, 2-[(5/-(4//-substituted phenyl)-1/ -phenyl) pyrazolyl] pyrroles III (a-m).The compounds were characterized by means of IR, 1H NMR and elemental analysis.
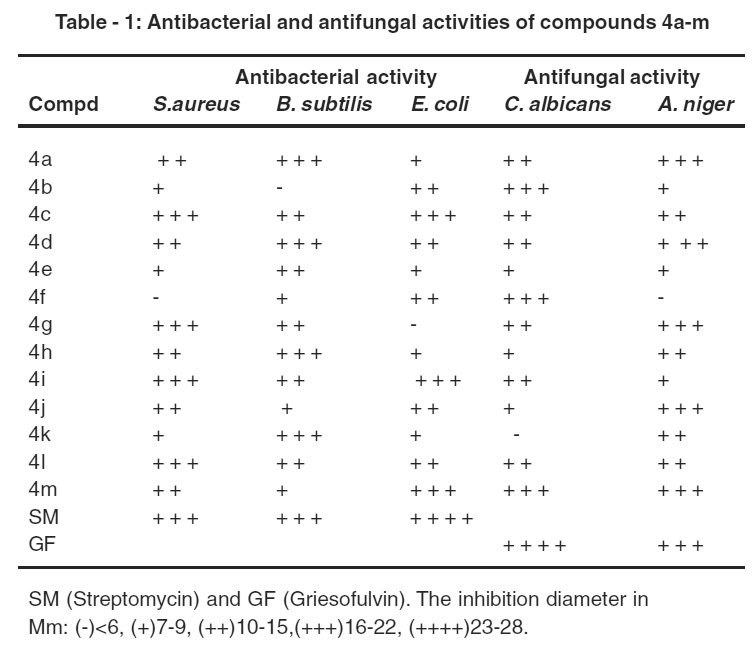 |
Table 1: Antibacterial and antifungal activities of compounds 4a-m Click here to view table |
Experimental Section
All the melting points were determined in open capillaries and are uncorrected. The IR spectra were run in KBr on a Perkins - Elmer infrared spectrophotometer.1 H NMR spectra on Bucker AC – 300F (300 Hz) NMR spectrometer using DMSO as a solvent using tetramethyl silane as internal standard
Synthesis of III from II
By Microwave Irradiation Method:
A) Solid Phase MWI
A solution of II (0.01mol), Phenyl hydrazine in ethanol (2ml) was taken in a 100ml borosil flask and to this KOH (1g) and basic alumna (3g) was added. The reaction mixture was thoroughly mixed, dried in air and irradiated inside a microwave oven for 5-7 min. at power level(700W), the reaction mixture was cooled. and extracted with ethanol (3x10ml).The resultant solid was recrystallized using aqueous ethanol.
B) Solution Phase MWI
Equimolar quantities of II, Phenyl hydrazine (0.01mol) and KOH in ethanol (30ml) were taken in a 100 ml borosil flask fitted with a funnel as a loose top. The reaction mixture was irradiated in a microwave oven for 5-6 min. at 20% power level (300W) with short interruption of 20 sec, to avoid the excessive evaporation of the solvent. This protocol was repeated in overall heating time. On completion of the reaction (TLC) the reaction mixture was cooled and acidified with dil HCl. The product 5 separated was filtered, washed with cold water, dried and recrystallized from ethanol.
Antimicrobial Activity
The compounds III a-m were screened for their antibacterial activity against Bacillus subtilis, staphylococcus aureus and Escherichia coil and antifungal activity against Candida albicans and Aspergillus nigar by filter paper disc techniquc.19 Standard antibacterial Streptomycin and antifungal Griscofulvin were also tested under similar conditions for comparison. Results are presented in Table -1.
Synthesis of 2-[substituted benz-1/(propene-1//-one)] Pyrroles II (a-m)
2-acetyl pyrrole (0.01mol) and 2- [substituted benz-1/(propene-1//-one)] Pyrroles II (0.01mol) was dissolved in 100ml ethanol. To this solution, NaOH (40%, 10ml) was added drop wise with constant stirring at room temp. till a dark yellow mass was obtained. The reaction mixture was kept 7-8 hr and acidified with dil HCl. The solid obtained was washed with cold water. It was filtered, dried and crystallized from ethanol. These compounds (2a-m) are synthesized by classical as well as microwave assisted reaction (The paper is in press).
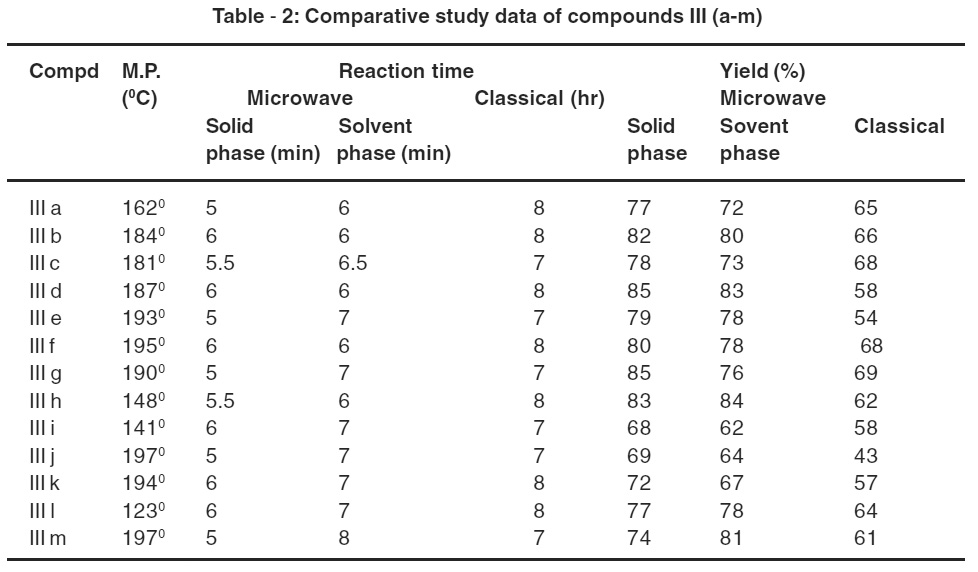 |
Table 2: Comparative study data of compounds III (a-m) Click here to view table |
Synthesis of 2-[(5/-(substituted phenyl)-1/ - phenyl) pyrazolyl] pyrroles III (a-m)
A mixture of benzylidene acetyl pyrroles II (0.01mol) and phenyl hydrazine (0.03mol) was refluxed in ethanol for 8 hr. The contents were evaporated to dryness and the product so obtained was washed with water repeatedly and then recrystallized from ethanol.
III a: 2-[(1/, 5/- phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Yield 65%, M.Pt.162°C:IR (KBr); 3335, (NH-pyrrole), 3044cm-1 (CH of pyrrole-) 1585cm-1 (C-N);1HNMR (DMSO-d6) 8.7 (1H, s, NH-pyrrole), 7.1 (Ar- H).
III b: 2-[(5/-(2//-hydroxy phenyl)-1/ -phenyl) pyrazolyl] pyrroles.
Yield 66%, M.P. 184°C; IR (KBr); 3422 cm-1 (-OH), 3337 (NH-pyrrole), 1547cm-1 (C-N), 3044cm-1 (CH of pyrrole-); 1HNMR (DMSO-d6) 9.2 (1H, s, NH-pyrrole), 5.7 (s, 1H, OH), 6.8 (Ar- H).
III c: 2-[(5/-(3//-hydroxy phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Yield 68%, M.P. 181°C: IR(KBr); 3420(-OH), 3440(NH-pyrrole), 3121(CH-pyrrole), 1550(C=N), 1511(Ar-H); 1HNMR (DMSO- d6) 8.7 (s, 1H, NHpyrrole), 6.8 (s, 1H, CH-pyrrole), 5.6 (s, 1H, OH), 7.6 (Ar- H).
III d: 2-[(5/-(4//-hydroxy phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Yield 58%, M.P187°C; IR (KBr); 3427(-OH), 3337cm-1 (NH-pyrrole), 1545cm-1 (C-N), 3143cm-1 (CH of pyrrole ); 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 5.5 (s, 1H, OH), 6.3-7.1 (Ar- H).
III e: 2-[(5/-(2//-nitro phenyl)-1/ -phenyl) pyrazolyl] pyrroles.
Yield 54%, M.P. 193° C; IR (KBr); 3325 (NH-pyrrole), 1577cm-1 (C-N), 3044cm-1 (CH of pyrrole-), 742cm-1(C NO2) ; 1HNMR (DMSO-d6) 8.2 (1H, s, NHpyrrole), 6.9 (Ar- H).
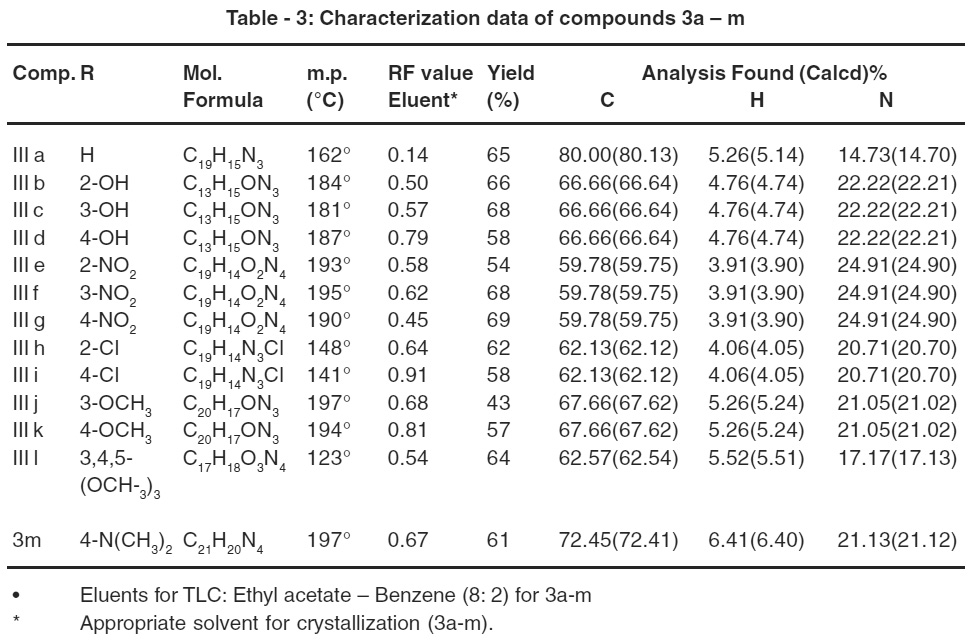 |
Table 3: Characterization data of compounds 3a – m Click here to view table |
III f: 2-[(5/-(3//-nitro phenyl)-1/ -phenyl) pyrazolyl] pyrroles.
Yield 68%, M.P. 195° C; IR (KBr); 3335 (NH-pyrrole), 1559cm-1 (C-N), 3044cm-1 (CH of pyrrole-) 746cm-1(C NO2); 1HNMR (DMSO-d6) 8.7 (1H, s, NHpyrrole), 7.6 (Ar- H).
III g: 2-[(5/-(4//-nitro phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Yield 69%, M.P. 190° C; IR (KBr) ; 3335 (NH-pyrrole), 1683 (C = 0), 1587cm-1 (C-N), 3044cm-1 (CH of pyrrole ), 744cm-1(C-NO2); 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 6.3-7.1 (Ar- H).
III h: 2-[(5/-(2//-chloro phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Yield 62%, M.P. 148° C; IR (KBr) ; 3335 (NH-pyrrole), 1683 (C = 0), 1548cm-1 (C-N), 3044cm-1 (CH of pyrrole-), 724cm-1(C-Cl); 1HNMR (DMSO-d6) 9.5 (1H, s, NH-pyrrole), 6.3 (Ar- H).
III i: 2-[(5/-(4//-chloro phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Yield 58%, M.P. 141° C; IR (KBr); 3335 (NH-pyrrole), 1587cm-1 (C-N), 3044cm-1 (CH of pyrrole-), 733cm-1(C-Cl); 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 6.3-7.1 (Ar- H).
III j: 2-[(5/-(3//-methoxy phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Yield 57%, M.P. 197° C; IR (KBr) ; 3335 (NH-pyrrole), 1587cm-1 (C-N), 3044cm-1 (CH of pyrrole-); 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 6.3-7.1 (Ar- H).
III k: 2-[(5/-(4//-methoxy phenyl)-1/ -phenyl) pyrazolyl] pyrroles
Yield 57%, M.P. 194° C; IR (KBr) ; 3335 (NH-pyrrole), 1587cm-1 (C-N), 3044cm-1 (CH of pyrrole-) ; 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 6.3-7.1 (Ar- H).
III l: 2-[(5/-(3//, 4//, 5//-trimethoxy phenyl)-1/ - phenyl) pyrazolyl] pyrroles
Yield 64%, M.P. 123° C; IR (KBr) ; 3335 (NH-pyrrole), 1587cm-1 (C-N), 3044cm-1 (CH of pyrrole-) ; 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 6.3-7.1 (Ar- H).
III m: 2-[(5/-(4//-dimethyl-amine phenyl)-1/ - phenyl) pyrazolyl] pyrroles
Yield 61%, M.P. 197° C; IR (KBr) ; 3335 (NH-pyrrole), 1587cm-1 (C-N), 3044cm-1 (CH of pyrrole-); 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 6.3-7.1 (Ar- H).
Conclusion
A series of III (a-m) were synthesized by the cyclisation of compound II (a-m) by phenyl hydrazine and KOH under solid supported microwave irradiations affording 73-92% yield within 7-8 min of reaction time period as compared to classical method. Solid sup portal microwave irradiation technique for carrying out organic reactions has been proved to be an efficient and environmentally benign methodology in terms of limited consumption of organic solvents, lesser reaction time and excellent yield of products.
Acknowledgements
Financial support from the UGC, New Delhi (project No. F 12-17, 2004, SR) is gratefully acknowledged.
References
- Kidwai M, Dave B J and Venkataramanan R, Indian J. Chem., (2002) 41B, 2414.
- Kidwai M, Rastogi S and Vekantaramanan R, Bull chem. Soc Jpn, (2003) 76, 203.
- Kidwai M & sapra P, Bhushan K R & Misra P, Synthesis, (2001) 10: 1509.
- Kidwai M & sapra P, Synth Commun. (2002) 32, 1639.
- Solid supports and catalysis in organic synthesis, edited by K . Smith (Prentice Hall, Chicherter) (1992).
- Clark J. H, Gullen S R, Barlow S J & Bastock T W. J. Chem. Soc, Perkin Trans (1994) 2, 1117.
- Kidwai M& kumar P, J. Chem. Research, (1996) 254.
- Kidwai M & Kohali P, Indian J Chem., 36, (1997) 1071.
- Bose A K, Banic Lavinskaia N, Jayaraman M & Manhas M S, Chemtech, (1997) 27, 18.
- Mitra A K, De A & karchaudhuri N, synth commun, 29, 1997, 573: Indian J Chem., (2000) 39B 311, 387.
- Loupy A, petit A, Hamclin J, Texier – Boullet F, Jacquault P and Mathe D, Synthesis, (1998) 1213.
- Texier –Boullet F, latouche R & Hamclin J, Tetrahedron Lett, (1997) 34, 2123.
- Loupy A, Spectra Analyses, (1993) 175, 33.
- Caddick S, Tetrahedron, (1995) 51, 10403.
- Oza H B, Joshi D G & Parikh H H, Heterocyclic comm., (1997) 3, 3.
- Kumar R S & Jaju B P, J Indian J.Indian chem. Soc., (1990) 67: 920.
- Tripathi S & Pandey B R, Indian J. pharmacol, 24, 1980,155.
- Wright J B, William E D & John H Markille, J Med chem., (1964) 7, 102.
- Weber A. D, Sanders C. S., Antimicrob agents Chemother, (2001) 34, 156.






