Prospects of Low-cost Chitosan as an Eco-friendly and Economic Water Purification Method
Haritma Chopra1
 , Drushti Sable2
, Drushti Sable2
 , Annu Kumari2
, Annu Kumari2
 , Anamika Sharma2
, Anamika Sharma2
 , Rishika Maji2
, Rishika Maji2
 , Preena Chahar2
, Preena Chahar2
 , Rakhi Gupta2
, Rakhi Gupta2
 and Archana Aggarwal2
and Archana Aggarwal2

1
Chemistry Department,
Maitreyi College,
University of Delhi,
Delhi
India
2
Zoology Department,
Maitreyi College,
University of Delhi,
Delhi
India
Corresponding author Email: aaggarwal@maitreyi.du.ac.in
Copy the following to cite this article:
Chopra H, Sable D, Kumari A, Sharma A, Maji R, Chahar P, Gupta R, Aggarwal A. Prospects of Low-cost Chitosan as an Eco-friendly and Economic Water Purification Method. Curr World Environ 2025;20(1).
Copy the following to cite this URL:
Chopra H, Sable D, Kumari A, Sharma A, Maji R, Chahar P, Gupta R, Aggarwal A. Prospects of Low-cost Chitosan as an Eco-friendly and Economic Water Purification Method. Curr World Environ 2025;20(1).
Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2025-02-04 |
|---|---|
| Accepted: | 2025-04-17 |
| Reviewed by: | 
 Vijaya Kumara
Vijaya Kumara
|
| Second Review by: |

 Keerthiga Gopalram
Keerthiga Gopalram
|
| Final Approval by: | Dr. Mohammad Rafatullah |
Introduction
Water serves as a vital solvent crucial for the survival and existence of life on Earth; however, this essential resource has been contaminated by human interference and has a greater risk to health and general well-being. There is continuous scarcity and decline in potable water quality, leading to greater health risks to the younger population, including water-borne infections.1
According to the UN 2019 World Water Development Report, there is a 20-30 % increase in demand for water for industrial and domestic purposes.2 Thus, it is imperative to conserve water resources and develop more eco-friendly and economical water purification strategies.
Multiple water treatment/purification methods have been studied using organic/inorganic polymers with efficient flocculating properties, but most are synthetic and non-biodegradable.3
Therefore, it is crucial to search for an incubation medium that is natural, non-toxic, biodegradable, biocompatible and readily available.
Chitosan is a naturally occurring biopolymer derived from the exoskeletons of marine crustaceans, such as crabs, prawns, lobsters, shrimps etc. This substance is a byproduct of seafood processing and is generated in significant quantities in coastal regions.
Chitosan exhibits a combination of properties required for water purification and has been used to remove organic pollutants, heavy metals, bacteria etc.4 Unlike most of the naturally occurring polysaccharides (cellulose, starch, alginate), having anionic or neutral charge, acid-soluble Chitosan is polycationic and can neutralise several ionic impurities like arsenic, molybdenum, cadmium, lead, copper, zinc, nickel and cobalt.5-9 Furthermore, as a coagulant, Chitosan has been reported to remove algal turbidity and turbidity from seawater and microbial harvesting in the laboratory.10-12 A recent study also suggested that modified Chitosan flocculants exhibited high purification efficiency in treating oil spilt water in a wide pH range.13
Chitosan and Chitosan palm membranes have demonstrated superior effectiveness in wastewater treatment compared to other commercially available membranes and expensive activated carbon.14 Furthermore, utilizing a natural coagulant such as Chitosan results in significant reductions in chemical usage and sludge management.15 Chitosan has proven to be an efficient coagulant for the treatment of tap water, with research indicating its capability to remove both particulate and dissolved solids.16,17 However, it is less efficient for dissolved organic carbon when incubated alone with water.18 This study aims to assess water quality and evaluate the performance of low-cost Chitosan as a more economical and dependable method for water purification in comparison to traditional techniques.
Materials and Methods
Sample Collection
Samples were collected from Geeta Colony, Delhi (Sample 1- RO wastewater), (Sample 2- Tap water) in pre-labelled 1 L polyethylene sampling bottles with no air trapped. Sampling bottles were rinsed in advance with the water sample intended for collection, and the water was permitted to flow for several minutes at the collection source prior to filling the bottles. Three sets of water samples from each of the water sources were collected. The sample bottles were then brought to the laboratory and treated with different Chitosan concentrations to check their effectiveness.
Chemicals
All the general chemicals and reagents used were of AR grade unless otherwise specified. Chitosan was procured from Merck. The Chitosan used had a Deacetylation Degree of a minimum of 90 % and viscosity of 150-500 mPa.
Preparation of Stock Solution of Chitosan
10 mL of 0.1 M HCl was added in 100 mg Chitosan with continuous stirring and incubation was done for 1 hour at room temperature (RT). The stock solution of 1 mg/mL was prepared by adding distilled water to achieve a final volume of 100 mL. The stock solution was further used to obtain desired concentrations for dose kinetics.
Physicochemical Analysis
Both the water samples were assessed for the water quality parameters, following classical laboratory methods and standard titrimetric procedures (Potentiometric titration with silver nitrate for Chloride; EDTA titrimetric method for total hardness).19 The colorimetric method was used for sulphate. All the parameters were tested in triplicates from each sample. Hardness was calculated using the Total hardness test kit (range: 5-100, 25-500 mg/L as CaCO3; Power Max Engineers).
Dose and Time Kinetic Study
Dose and time kinetics study was conducted with both the samples. 500 mL water samples (1 and 2) were incubated with various doses (8 mg/L, 20 mg/L) of Chitosan in pre-labelled conical flasks at room temperature. An aliquot of 100 mL water sample was collected from the treated samples at 0 h, 1 h, 2 h, 4 h and 24 h, respectively, for physio-chemical analysis at room temperature.
Statistical Analysis
All experiments were conducted on three separate occasions, and each figure presented the outcomes of three independent experiments that yielded comparable results. Statistical analysis was performed utilizing a two-tailed Student’s t-test, with results deemed statistically significant at p < 0.05.
Results
Contaminants in water pose significant challenges to water treatment processes and the environment. The present study focused on using low-cost Chitosan as an efficient way of removing impurities from water samples taken from a selected site of Delhi.
Analysis of TDS level
It was analysed that before treatment, both the samples had very high TDS levels (sample 1-2050±0 mg/L and sample 2-1960±0 mg/L), almost four times the permissible range as given by WHO; i.e. 500 mg/L. With an increase in Chitosan dose and the incubation time, a decrease in TDS was observed in both samples.
The values for TDS were 1986.67±5.77 mg/L (p<0.01) and 1756.67±5.77 mg/L (p<0.001) with 20 mg/L after 24 h of incubation in sample 1 and sample 2 respectively (Figure 1A, 1B; Table 1). Although the changes observed in the TDS value were statistically significant for both samples, the downregulation was not as per the permissible range of drinking water.
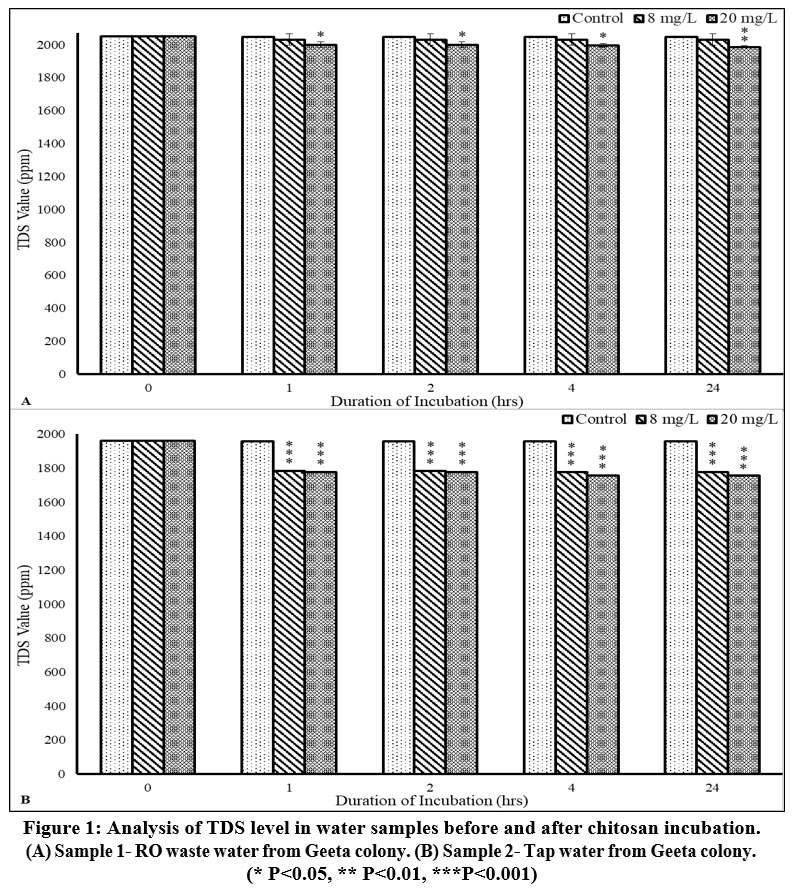 | Figure 1: Analysis of TDS level in water samples before and after chitosan incubation. (A) Sample 1- RO waste water from Geeta colony. (B) Sample 2- Tap water from Geeta colony.
|
Table 1: Analysis of TDS level in water samples before and after chitosan incubation (* P<0.05, ** P<0.01, ***P<0.001).
Sample 1 (RO Waste Water from Geeta Colony) | |||
Concentration of Chitosan (mg/L) | Duration of Incubation (h) | TDS Value (ppm) | p Value |
0 (Control) | 0 | 2050±0 | |
1 | 2046.67±5.77 | 0.42 | |
2 | 2046.67±5.77 | 0.42 | |
4 | 2046.67±5.77 | 0.42 | |
24 | 2046.67±5.77 | 0.42 | |
8 | 0 | 2050±0 | |
1 | 2030±34.64 | 0.42 | |
2 | 2030±34.64 | 0.42 | |
4 | 2030±34.64 | 0.42 | |
24 | 2030±34.64 | 0.42 | |
20 | 0 | 2050±0 | |
1 | 2000±17.32* | 0.04 | |
2 | 2000±17.32* | 0.04 | |
4 | 1996.67±11.55* | 0.02 | |
24 h | 1986.67±5.77** | 0.3 X 10-2 | |
Sample 2 (Tap Water from Geeta Colony) | |||
Concentration of Chitosan (mg/L) | Duration of Incubation (h) | TDS Value (ppm) | p Value |
0 (Control) | 0 | 1960±0 | |
1 | 1956.67±5.77 | 0.42 | |
2 | 1956.67±5.77 | 0.42 | |
4 | 1956.67±5.77 | 0.42 | |
24 | 1956.67±5.77 | 0.42 | |
8 | 0 | 1960±0 | |
1 | 1783.33±5.77*** | 0.4 X 10-3 | |
2 | 1783.33±5.77*** | 0.4 X 10-3 | |
4 | 1776.67±5.77*** | 0.3 X 10-3 | |
24 | 1776.67±5.77*** | 0.3 X 10-3 | |
20 | 0 | 1960±0 | |
1 | 1776.67±5.77*** | 0.3 X 10-3 | |
2 | 1776.67±5.77*** | 0.3 X 10-3 | |
4 | 1756.67±5.77*** | 0.3 X 10-3 | |
24 | 1756.67±5.77*** | 0.3 X 10-3 | |
Analysis of hardness level
Before treatment, both the samples had hardness above the permissible range. The hardness levels in both the samples were observed to be 1483.33±28.87 mg/L and 1016.67±28.87 mg/L, making them very hard and unsuitable for drinking. In sample 1, the hardness decreased to 583.33±28.87 mg/L (p<0.001), and in sample 2, the hardness decreased to 500±0 mg/L (p<0.01) after incubation with 20 mg/L dose of Chitosan for 1 hour (Figure 2A, 2B; Table 2). Therefore, a considerable decrease was observed in hardness levels of both the samples but are still not suitable for drinking.
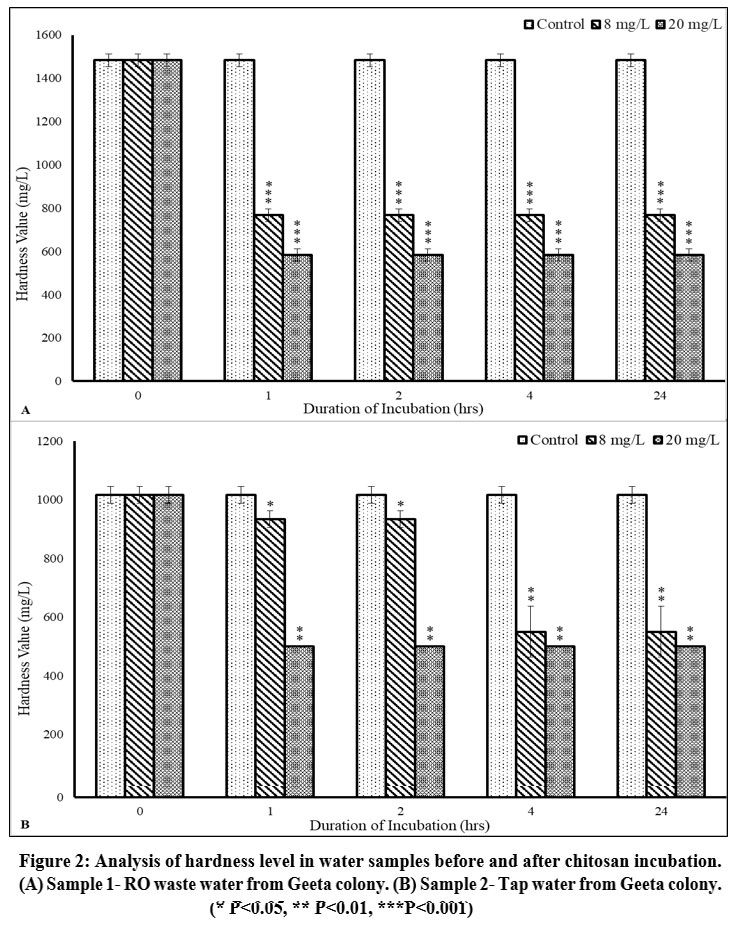 | Figure 2: Analysis of hardness level in water samples before and after chitosan incubation. (A) Sample 1- RO waste water from Geeta colony. (B) Sample 2- Tap water from Geeta colony.
|
Table 2: Analysis of hardness level in water samples before and after chitosan incubation (* P<0.05, ** P<0.01, ***P<0.001).
Sample 1 (RO Waste Water from Geeta Colony) | |||
Concentration of Chitosan (mg/L) | Duration of Incubation (h) | Hardness Value (mg/L) | p Value |
0 (Control) | 0 | 1483.33±28.87 | |
1 | 1483.33±28.87 | 1 | |
2 | 1483.33±28.87 | 1 | |
4 | 1483.33±28.87 | 1 | |
24 | 1483.33±28.87 | 1 | |
8 | 0 | 1483.33±28.87 | |
1 | 766.67±28.87*** | 0.7 X 10-5 | |
2 | 766.67±28.87*** | 0.7 X 10-5 | |
4 | 766.67±28.87*** | 0.7 X 10-5 | |
24 | 766.67±28.87*** | 0.7 X 10-5 | |
20 | 0 | 1483.33±28.87 | |
1 | 583.33±28.87*** | 0.3 X 10-5 | |
2 | 583.33±28.87*** | 0.3 X 10-5 | |
4 | 583.33±28.87*** | 0.3 X 10-5 | |
24 | 583.33±28.87*** | 0.3 X 10-5 | |
Sample 2 (Tap Water from Geeta Colony) | |||
Concentration of Chitosan (mg/L) | Duration of Incubation (h) | Hardness Value (mg/L) | p Value |
0 (Control) | 0 | 1016. 67±28.87 | |
1 | 1016. 67±28.87 | 1 | |
2 | 1016. 67±28.87 | 1 | |
4 | 1016. 67±28.87 | 1 | |
24 | 1016. 67±28.87 | 1 | |
8 | 0 | 1016. 67±28.87 | |
1 | 933. 33±28.87* | 0.02 | |
2 | 933. 33±28.87* | 0.02 | |
4 | 550±86.60** | 0.7 X 10-2 | |
24 | 550±86.60** | 0.7 X 10-2 | |
20 | 0 | 1016.67±28.87 | |
1 | 500±0** | 0.1 X 10-2 | |
2 | 500±0** | 0.1 X 10-2 | |
4 | 500±0** | 0.1 X 10-2 | |
24 | 500±0** | 0.1 X 10-2 | |
Analysis of chloride level
The chloride levels were almost double the permissible range of chloride for drinking water before treatment with Chitosan for both the samples (sample 1- 561.29±27.07 mg/L and sample 2- 543.27± 6.21 mg/L), thus making them highly corrosive. In sample 1, significant changes were observed after treatment with a 20 mg/L dose of Chitosan 387.45±23.16 mg/L (p<0.01) after 24 h of incubation. In sample 2, after treatment with Chitosan (20 mg/L dose), the chloride concentration decreased to 431.31±10.23 mg/L (p<0.001) after 4 h of incubation (Figure 3A, 3B; Table 3).
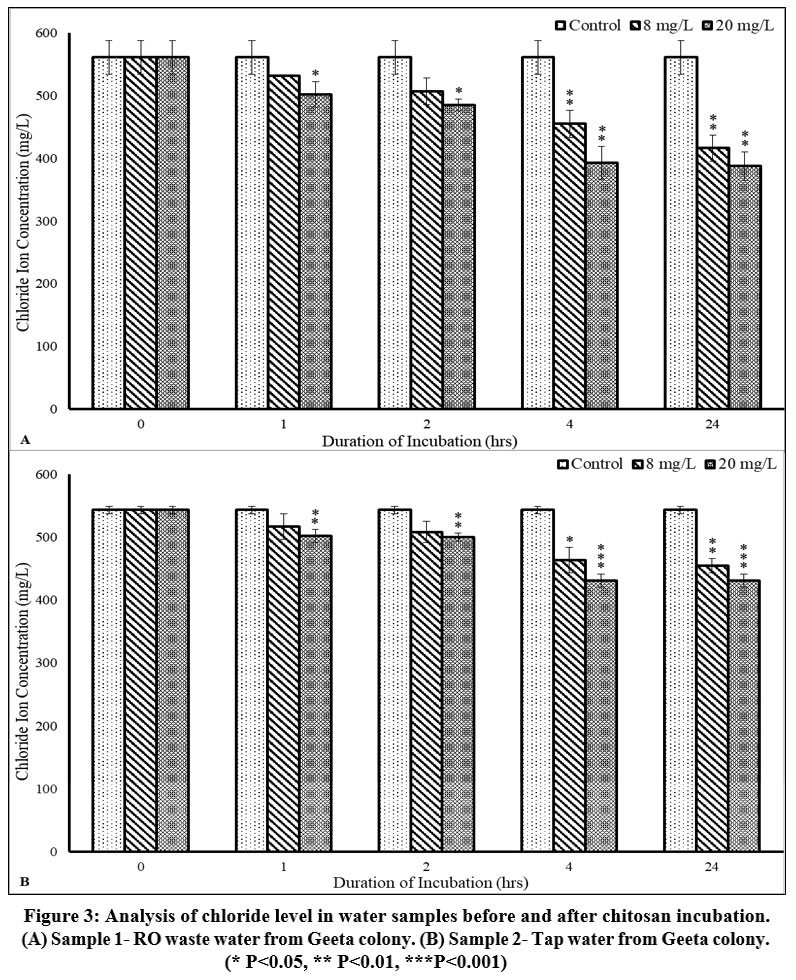 | Figure 3: Analysis of chloride level in water samples before and after chitosan incubation. (A) Sample 1- RO waste water from Geeta colony. (B) Sample 2- Tap water from Geeta colony.
|
Table 3: Analysis of chloride ion concentration in water samples before and after chitosan Incubation (* P<0.05, ** P<0.01, ***P<0.001).
Sample 1 (RO Waste Water from Geeta Colony) | |||
Concentration of Chitosan (mg/L) | Duration of Incubation (h) | Chloride Ion Concentration (mg/L) | p Value |
0 (Control) | 0 | 561.29±27.07 | |
1 | 561.29±27.07 | 1 | |
2 | 561.29±27.07 | 1 | |
4 | 561.29±27.07 | 1 | |
24 | 561.29±27.07 | 1 | |
8 | 0 | 561.29±27.07 | |
1 | 531.75±0 | 0.20 | |
2 | 507.07±21.39 | 0.06 | |
4 | 455.14±21.67** | 0.7 X 10-2 | |
24 | 416.54±20.90** | 0.2 X 10-2 | |
20 | 0 | 561.29±27.07 | |
1 | 502.21±20.47* | 0.04 | |
2 | 485.33±9.58* | 0.03 | |
4 | 392.51±26.26** | 0.1 X 10-2 | |
24 | 387.45±23.16** | 0.1 X 10-2 | |
Sample 2 (Tap Water from Geeta Colony) | |||
Concentration of Chitosan (mg/L) | Duration of Incubation (h) | Chloride Ion Concentration (mg/L) | p Value |
0 (Control) | 0 | 543.27±6.21 | |
1 | 543.27±6.21 | 1 | |
2 | 543.27±6.21 | 1 | |
4 | 543.27±6.21 | 1 | |
24 | 543. 27±6.21 | 1 | |
8 | 0 | 543. 27±6.21 | |
1 | 516.68±20.38 | 0.14 | |
2 | 508.51±16.76 | 0.06 | |
4 | 463.51±20.39* | 0.02 | |
24 | 454.64±11.52** | 0.1 X 10-2 | |
20 | 0 | 543.27±6.21 | |
1 | 502.21±10.23** | 0.73 X 10-2 | |
2 | 500.24±6.82** | 0.13 X 10-2 | |
4 | 431.31±10.23*** | 0.3 X 10-3 | |
24 | 431.31±10.23*** | 0.3 X 10-3 | |
Analysis of sulphate level
In untreated samples, sulphate levels were found to be relatively low, although they exceeded the permissible limits, which may result in an unpleasant taste and laxative effects.20 Prior to incubation with Chitosan, the sulphate concentrations measured were 422.53±0 mg/L for sample 1 and 380.28±0 mg/L for sample 2. Chitosan treatment resulted in a decrease in sulphate ion concentration in both the samples though the difference was observed to be relatively low. The sulphate concentration in sample 1 was 396.71±4.07 mg/L (p<0.01), after incubation with 20 mg/L dose of Chitosan for 24 h, while in sample 2, the concentration of sulphate declined to 337.67±14.44 mg/L (p<0.05) after 24 h of incubation at 8 mg/L dose of Chitosan (Figure 4A, 4B; Table 4).
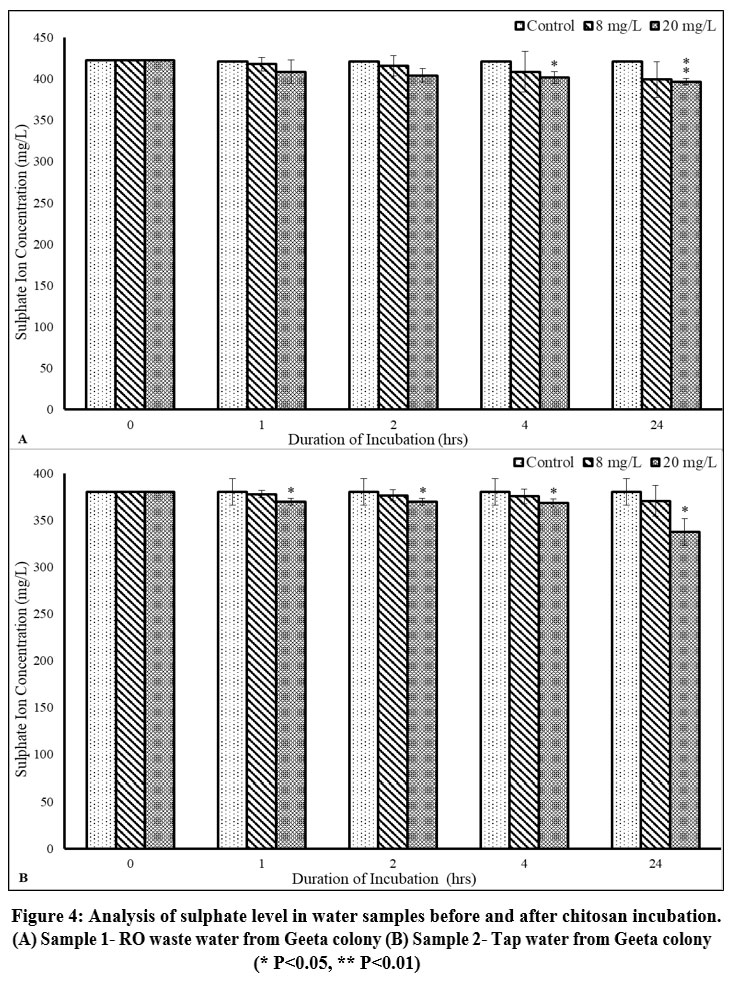 | Figure 4: Analysis of sulphate level in water samples before and after chitosan incubation. (A) Sample 1- RO waste water from Geeta colony (B) Sample 2- Tap water from Geeta colon
|
Table 4: Analysis of sulphate ion concentration in water samples before and after chitosan Incubation (* P<0.05, ** P<0.01, ***P<0.001).
Sample 1 (RO Waste Water from Geeta Colony) | |||
Concentration of Chitosan (mg/L) | Duration of Incubation (h) | Sulphate Ion Concentration (mg/L) | p Value |
0 (Control) | 0 | 422.53±0 | |
1 | 421.01±2.63 | 0.42 | |
2 | 421.01±2. 63 | 0. 42 | |
4 | 421.01±2. 63 | 0. 42 | |
24 | 421.01±2. 63 | 0. 42 | |
8 | 0 | 422.53±0 | |
1 | 417.84±8.13 | 0. 42 | |
2 | 415.49±12.20 | 0. 42 | |
4 | 408.45±24.39 | 0. 42 | |
24 | 399.06±21.51 | 0.20 | |
20 | 0 | 422.53±0 | |
1 | 408.45±14.09 | 0.23 | |
2 | 403.82±8.19 | 0.06 | |
4 | 401.41±7.04* | 0.04 | |
24 | 396.71±4.07** | 0.8 X 10-2 | |
Sample 2 (Tap Water from Geeta Colony) | |||
Concentration of Chitosan (mg/L) | Duration of Incubation (h) | Sulphate Ion Concentration (mg/L) | p Value |
0 (Control) | 0 | 380.28±0 | |
1 | 380.28±14.09 | 1 | |
2 | 380. 28±14.09 | 1 | |
4 | 380. 28±14.09 | 1 | |
24 | 380. 28±14.09 | 1 | |
8 | 0 | 380.28±0 | |
1 | 377.93±4.07 | 0.42 | |
2 | 376.76±6.10 | 0.42 | |
4 | 375.58±8.14 | 0.42 | |
24 | 370.89±16.26 | 0.42 | |
20 | 0 | 380.28±0 | |
1 | 369.71±3.52* | 0.03 | |
2 | 369.71±3.52* | 0.03 | |
4 | 368.54±4.07* | 0.03 | |
24 | 337.67±14.44* | 0.03 | |
Discussion
The utilization of low-cost Chitosan, a biodegradable and biocompatible polymer, for its water purification capabilities is crucial, particularly in India, where a significant portion of the population lives below the poverty line. This approach offers a more economical alternative to traditional water treatment methods.
Water quality parameters serve as critical indicators for assessing the appropriateness and safety of water for diverse applications. Some of the key parameters include total dissolved solids (TDS), water hardness, chloride ions, and sulphate ions. The samples that were collected for the present study were highly unsuitable for drinking purposes, with all the parameters crossing the guidelines as proposed by WHO, 2011.
Total Dissolved Solids (TDS) consists of inorganic minerals such as calcium, magnesium, potassium, and sodium, along with trace amounts of organic substances. This parameter is crucial for evaluating the potability of water, as a specific concentration of these ions is necessary for safe drinking water. However, water with elevated TDS levels often lacks aesthetic qualities, particularly in terms of taste and odor. Additionally, high TDS water may have a laxative effect, potentially leading to negative reactions in individuals sensitive to this condition.21 Though elevated TDS levels are not life-threatening, but they may pose risks for those with pre-existing kidney and heart conditions.22 In the present study, Chitosan was found to be more effective at 20 mg/L concentration than 8 mg/L. The effectiveness of Chitosan was observed by its purification efficiency for various parameters. There was a 3.09 % and 10.37 % decline in TDS level after treatment with Chitosan in samples 1 and 2, respectively.
Water hardness is a general indicator of water quality and represents compounds of calcium and magnesium dissolved in water and in some cases other divalent and trivalent metal elements. According to WHO guidelines (2011), water with a concentration of less than 60 mg/L is categorized as soft; concentrations ranging from 60 to 120 mg/L are deemed moderately hard; those between 120 and 180 mg/L are classified as hard; and water exceeding 180 mg/L is considered very hard. Moderately hard water adds to the dietary calcium and magnesium, thus having health benefits. However, water with hardness above 200 mg/L is unacceptable for drinking and domestic purposes.23 The incubation process involving chitosan resulted in a reduction of hardness by 60.67% for sample 1 and 50.82% for sample 2, which is very satisfactory.
Chloride ions occur naturally in both surface and groundwater sources. It increases the electrical conductivity of water and thus increases its corrosivity. According to WHO guidelines (2011), the permissible limit for chloride concentrations in public drinking water is set at a maximum of 250 mg/L.24 However, chloride levels may be higher in drinking water due to water treatment with chlorine as a cleaner and may cause salty taste & skin irritation. Congestive heart failure or hypertension is not solely caused by an excess of chloride, but rather by the metabolism of sodium chloride.24 The treatment of chitosan led to a purification efficiency of 30.97% for sample 1 and 20.61% for sample 2 at a concentration of 20 mg/L over an incubation period of 24 h for chloride ion concentration.
Sulphate can originate from natural sources or result from discharges from municipal or industrial activities. At typical concentrations, sulphates are not deemed toxic. However, elevated levels of sulphate can impart a bitter or medicinal flavor to water and may cause laxative effects, potentially leading to dehydration due to diarrhea.23 The World Health Organization (WHO) guidelines from 2011 stipulate that a maximum concentration of 250 mg/L of sulphate in drinking water is acceptable.24 The concentration of sulphate ions decreased by 6.67% in sample 1, whereas sample 2 experienced a decline of 11.20% when water samples were co-incubated with chitosan at a concentration of 20 mg/L for 24 h. Chitosan was found to be an effective coagulating and flocculating agent for drinking water treatment. There was a significant decrease in all the four physicochemical parameters, namely TDS, Total Hardness, Chloride and Sulphate ions.
The purification efficiency of Chitosan in the present study represents a significant improvement in the water quality of RO wastewater (sample 1) with Chitosan. Thus, RO wastewater can potentially be used for domestic purposes, initially highly unsuitable, with high TDS and Hardness. It is an effective contribution to the purification of wastewater and water conservation.
Conclusion
Further standardization of Chitosan concentrations may help in successful water purification for drinking purposes. For better purification, more parameters can be tested with further standardization of Chitosan doses and biological analysis of the water samples. Additionally, a larger sample size is necessary to ensure broader public applicability. Even low-cost Chitosan alone has proven to be a water purifier, and in lower concentration, it is a more affordable system of water purification for the population belonging to the low socio-economic community. A suitable, cost-effective, easily accessible and economical water purifying kit can be prepared for the low-income class public.
Acknowledgment
The authors would like to acknowledge the help and support extended by the laboratory staff, Zoology Department, Maitreyi College, University of Delhi, Delhi. We would also thank to the Annual Research Project Scheme of Centre of Research, Maitreyi College, University of Delhi for giving us this research opportunity.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
Data sets in the form of tables
Ethics Statement
This study did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants and therefore, informed consent was not required.
Permission to reproduce material from other sources
Not Applicable
Author Contribution
Haritma Chopra: Conceptualization of the project, Supervision, Project Administration.
Archana Aggarwal: Conceptualization of the project, Methodology, Writing, Editing, Supervision, Project Administration.
Drushti Sable: Data Collection, Analysis, Writing.
Annu Kumari: Data Collection, Analysis.
Anamika Sharma: Data Collection, Analysis.
Rishika Maji: Data Collection, Analysis.
Preena Chahar: Data Collection, Analysis.
Rakhi Gupta: Writing & Editing
References
- WHO. Global Water Supply and Sanitation Assessment. World Health Organization. Geneva. 2000. Retrieved From: https://www.who.int/docstore/ water_sanitation_health/ Globassessment/Global1.htm#
- UNESCO World Water Assessment Programme, The United Nations World Water Development Report 2019: Leaving No One Behind. United Nations Education, Scientific & Cultural Organization. 2019, Retrieved From: https://unesdoc.unesco.org/ark:/48223/pf0000367306
- Chopra H., Ruhi G. Eco friendly Chitosan: An efficient material for water purification. The Pharma Innovation Journal. 2016;5(1):92-95. Retrieved from: https://www.thepharmajournal.com/archives/?year=2016&vol=5&issue=1&ArticleId=726
- Vijayan V. An Experimental Study on Chitosan for Water Treatment. Int. J. Curr. Adv. Res. 2018;7(5):12242-12247. DOI: http://dx.doi.org/10.2139/ssrn.3591432
CrossRef - Tondwal R., Singh, M. Chitosan functionalization with a series of sulfur-containing ?-amino acids for the development of drug-binding abilities. J. Appl. Polym. Sci. 2017;135(12):46000. DOI: https://doi.org/10.1002/app.46000
CrossRef - Cumpstey I. Chemical modification of polysaccharides. ISRN Org. Chem. 2013;2013(1):417672. Retrieved from: https://onlinelibrary.wiley.com/doi/full/10.1155/ 2013/417672 DOI: https://doi.org/10.1155/2013/417672
CrossRef - Hassan M. A. A., Puteh M. H. Pre-treatment of palm oil mill effluent (POME): a comparison study using chitosan and alum. Malays. J. Civ. Eng. 2007;19(2):128-141. DOI: https://doi.org/10.11113/mjce.v19.15747
CrossRef - Thambiliyagodage C., Jayanetti M., Mendis A., Ekanayake G., Liyanaarachchi H., Vigneswaran S. Recent Advances in Chitosan-Based Applications-A Review. Material. 2023;16(5):2073. DOI: https://doi.org/10.3390/ma16052073
CrossRef - Karimi F., Ayati A., Tanhaei B., Sanati A.L., Afshar S., Kardan A., Dabirifar Z., Karaman C. Removal of Metal Ions Using a New Magnetic Chitosan Nano-Bio-Adsorbent; A Powerful Approach in Water Treatment. Environ. Res. 2022;203:111753. DOI: https://doi.org/10.1016/j.envres.2021.111753
CrossRef - Fast S. A., Kokabian B., Gude V. G. Chitosan enhanced coagulation of algal turbid waters – Comparison between rapid mix and ultrasound coagulation methods. Chem. Eng. J. 2014;244:403–410. DOI: https://doi.org/10.1016/j.cej.2014.01.081
CrossRef - Altaher H. The use of chitosan as a coagulant in the pre-treatment of turbid sea water. J. Hazard. Mate. 2012;233:97–102. DOI: https://doi.org/10.1016/j.jhazmat.2012.06.061
CrossRef - Ahmad A., Mat Yasin N., Derek C., Lim J., Optimization of microalgae coagulation process using chitosan. Chem. Eng. J. 2011;173(3):879–882. DOI: https://doi.org/10.1016/j.cej.2011.07.070
CrossRef - Ma J., Xia W., Zhang R., Ding L., Kong Y., Zhang H., Fu K. Flocculation of emulsified oily wastewater by using functional grafting modified chitosan: The effect of cationic and hydrophobic structure. J. Hazard. Mater. 2021;403:123-690. DOI: https://doi.org/10.1016/j.jhazmat.2020.123690
CrossRef - Alshahrani A. A., Alsuhybani M., Algamdi M. S., Alquppani D., Mashhour I., Alshammari M. S., Alraddadi T. S. Evaluating the performance of chitosan and chitosan-palm membrane for water treatment: preparation, characterization and purification study. J. Taibah Univ. Sci. 2021;15(1):77-86. DOI: https://doi.org/10.1080/16583655.2021.1885192
CrossRef - Diaz A., Rincon N., Escorihuela A., Fernandez N., Chacin E., Forster C. A preliminary evaluation of turbidity removal by natural coagulants indigenous to Venezuela. Process Biochem. 1999;35(3–4):391–395. DOI: https://doi.org/10.1016/S0032-9592(99)00085-0
CrossRef - Pontius F. W. Chitosan as a drinking water treatment coagulant. Am. J. Civ. Eng. 2016;4(5):205-215. DOI: http://10.11648/j.ajce.20160405.11
- Kangamaa A., Zeng D., Tian X., Fang J. Application of Chitosan Composite Flocculant in Tap Water Treatment. J. Chem. 2018;1-9. DOI: https://doi.org/10.1155/2018/2768474
CrossRef - Fabris R., Chow C. W., Drikas M. Evaluation of chitosan as a natural coagulant for drinking water treatment. Water Sci. Technol: A Journal of the International Association on Water Pollution Research. 2010;61(8):2119–2128.
CrossRef - WHO. Guidelines for drinking-water quality. World Health Organization. 1993. Retrieved from: https://apps.who.int/iris/bitstream/handle/10665/259956/ 9241544600- eng.pdf?sequence=1&isAllowed=y
- Bamini R. T. S., Thiruthani M., MP A. K. J., Balamani S. The purification and detoxification methods of Kanthakam (Sulphur)-A Siddha Review. J. Trad. Integrative Med. 2021;4(2):515-520. 20 DOI: http://10.1016/j.tim.2021
CrossRef - Biswas S., Kurup P. S. Study of essential and desirable characteristics and metal analysis of water samples of Maharashtra by using anionic surfactant. J. Industrial Control Pollution. 2012;28(2):155-162.
- Sasikaran S., Sritharan K., Balakumar S., Arasaratnam V. Physical, chemical and microbial analysis of bottled drinking water. The Ceylon medical journal. 2012;57(3):111–116.
CrossRef - Heizer W. D., Sandler R. S., Seal E., Jr, Murray S. C., Busby M. G., Schliebe B. G., Pusek S. N. Intestinal effects of sulfate in drinking water on normal human subjects. Digestive diseases and sciences. 1997;42(5):1055–1061. DOI: https://doi.org/10.1023/A:1018801522760
CrossRef - WHO. Guidelines for drinking-water quality. WHO Chronicle. 2011;38(4):104-108. Retrieved from:https://apps.who.int/iris/bitstream/handle/ 10665/44584/ 9789241548151_eng.pdf;jsess ionid=3D09EA72CFE0E96CAE157D39E60AF950?sequence=1






