Effect of pH on the removal of Victoria blue by adsorption
K.N. Singh1 * and Ranjana Singh1
1
Department of Chemistry,
S.G.R. (P.G.) College Dobhi,
Jaunpur,
222 149
India
DOI: http://dx.doi.org/10.12944/CWE.1.2.11
The removal of Victoria blue dye form water and waste water by adsorption on China clay is greatly influenced by the pH of the medium. The pH of the adsorbate solution effect the nature of the surface properties of adsorbents the degree of ionization and speciation of aqueous adsorbate species and ultimately the rate and extent of the adsorption. The initial concentration of the dye taken was 1.0 × 10-6 M and the China were agitated at 30° C (±°C). The pH of the dye solution was changed from 3.0 to 8.0 using NaOH or HCI solutions of appropriate strength. The results the variation in adsorption with different pH have been recorded.
Copy the following to cite this article:
Singh K.N, Singh R. Effect of pH on the removal of Victoria blue by adsorption. Curr World Environ 2006;(1):161-163 DOI:http://dx.doi.org/10.12944/CWE.1.2.11
Copy the following to cite this URL:
Singh K.N, Singh R. Effect of pH on the removal of Victoria blue by adsorption. Curr World Environ 2006;(1):161-163. Available from: http://www.cwejournal.org/?p=1024
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2006-10-29 |
|---|---|
| Accepted: | 2006-12-03 |
Introduction
Through various method viz. chemical oxidation, Froth flotation and coagulation have been employed for colour removal, adsorption offers the best for removal of wide ranging pollutants such as dissolved solids, heavy metals and organic dyes, Activated carbons was widely used as an adsorbent, though it is quite expensive which resuscitates to explore low cost adsorbents. The aim of this paper is to use cheaper adsorbent i.e. china clay to its high efficiency, easy handling and less expensiveness for the removal of Victoria blue from water.
Experimental
Batch adsorption experiments were performed in a shaking incubator by agitating different glass bottles containing 1.0 g of adsorbent in 50 ml aqueous solution of Victoria blue at desired concentration at 30° C (±0.1°C) and 3.0 to 8.0 pH. The pH of the dye solution was changed form 3.0 to 8.0 using NaOH or HCI of appropriate strength. The results showing the variation in adsorption and desorption of Victoria blue with corresponding pH has been recorded in Table -1 and is shown graphically in Fig. -1.
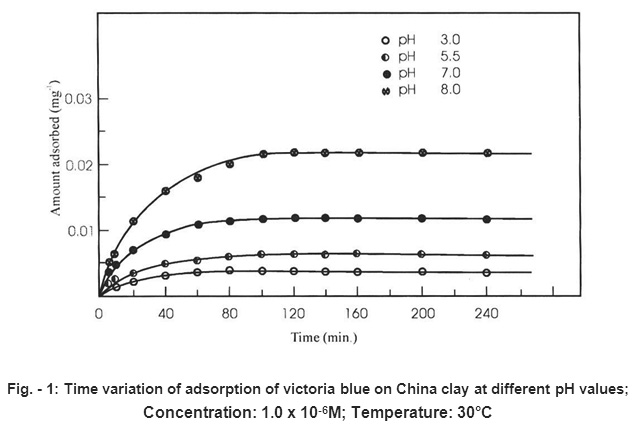 |
Figure - 1: Time variation of adsorption of victoria blue on China clay at different pH values; Concentration: 1.0 x 10-6M; Temperature: 30°C Click here to view figure |
Results and Discussion
It is evident form the results (i.e., table 1 and Fig. -1) that the pH of the medium has greatly influenced the uptake of Victoria blue by China Clay through the basis nature of time variation curve remains the same. The rate as well as the total amount of Victoria blue adsorbed at pre-equilibrium and equilibrium stages vary with change in pH of the solution to different extent. However, the time required to attain the equilibrium does not change to any appreciable extent with the change in pH. The amount of Victoria blue adsorbed (cf table-1 and Fig. -1) change from 0.0085 to 0.2160, the changed in pH from 3.0 to 8.0 at initial dye concentration of 1.0 × 10-6 M and temperature 30°C (± 0.1°C). Similar findings were reported earlier by several workers.
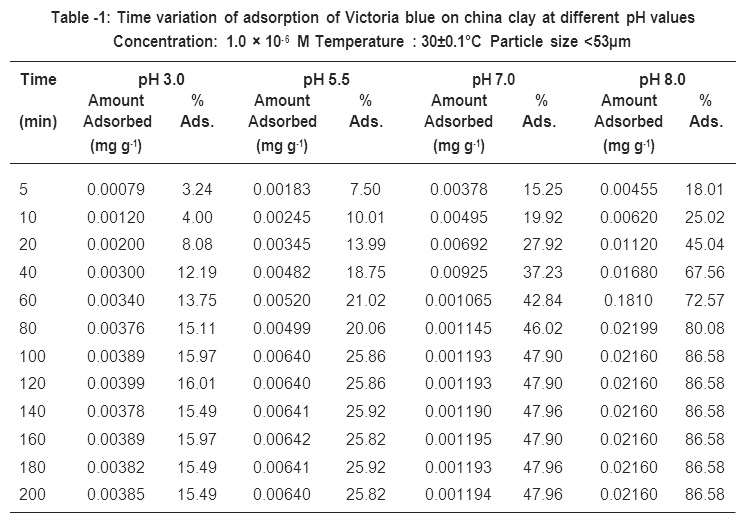 |
Table -1: Time variation of adsorption of Victoria blue on china clay at different pH values Concentration: 1.0 × 10-6 M Temperature : 30±0.1°C Particle size <53μm Click here to view table |
The increase in rate as well as the extent of adsorption of dye cations on China clay may be explained on the basis of the formation of surface hydroxyl components and their subsequent acid acid base dissociation which give the +ve or -ve charged surface depending upon the pH of the medium. The variation in amount absorbed at different pH Values can also be explained on the basis of change in surface electrical force and charged particles in the vicinity of the adsorbent. The hydroxylated oxide present in the China clay
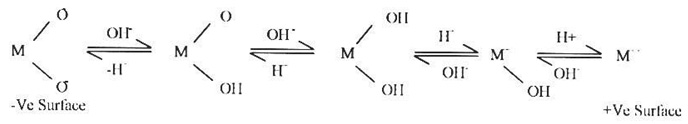
Where, M represent Al, Si and Ca etc. The adsorbents used in this present study mainly contain the oxides of silicon and Aluminum as alumino silicate (Si4Al4O10(OH)8). These oxide surfaces are expected to behave differently in acidic and alkaline medium due to their different surface charges and different types of orientations in the presence of varying amount of H+ and OH- ions with increase in pH of the solution, the surface negative charge increase and hence the extent of adsorption of Victoria blue cations increases with increase in pH. However, at lower pH, in acidic medium, the oxide develops electrical charges at the solids solution interface in the following manner. surface will be positively charged resulting in unfavorable conditions for the adsorption of dye cations. It has been suggested that the adsorption of cations at the solid solution interface is governed by the strongly adsorbed hydroxo, sulphato, carboylato and other metal species.
The enhanced removal of Victoria blue is mainly attributed to the presence of OH ions in the adsorbate solution in alkaline medium. Manzel and Coworkers have suggested that the presence of one hydroxyl ion would force one adsorbed cation to be more readily ammonodated in the surface lattice followed by further adsorption.
The zero point charges of alumina and Silica are 8.3 and 2.2 respectively. Thus, the surface of alumina in China clay possesses positive charge at low pH range, which is unfavorable for the interaction of cations.
The findings are quite useful in developing an appropriate technology for designing a waste water treatment plant where in such unconventional materials could be utilized as potential adsorbents.
Acknowledgements
We are thankful to Principal P.K. Singh. S.G.R. P.G. College Dhobi, Jaunpur for providing necessary facilities and Dr. A. Pradhan Deptt. of Mathematic, Hindu P.G. College, Zamania, Ghazipur for financial as well as moral support.
References
- Eckenfelder, W.W. Jr and O’Connor. D.J. “Biological waste treatment”, Pergman Press, New York (1961).
- Hudson, H.E. Jr., J. Amer Water Works Assoc. (1965) 57, 855.
- Bhargava, R.Mathur, R.P. and Khanna, P. Indian J. Env. Health, (1974) 16, 109.
- Pandey, K.K., Prasad, G. and Singh, V.N., Indian J. Chem. (1984) 23A 514.
- Mekay, G. Otterburn, M.S. and Jamal, A.A., Water, Air and Soil Pollut, (1985) 24, 307.
- Khare, S.K., Pandey, K.K, Srivastwa, R.M. and Singh, V.N., J. Chem. Tech. Biotechnol. (1987) 38, 99.
- Rai, A.K., Ph D. Thesis Banaras Hindu University, India (1995).
- Khatri, S.D. and Singh M.K., Indian J. Chem. Technology (1999) 6, 112.






