Effect of Rainwater Harvesting on the Amounts of Some Heavy Metals, in the Ground Water of East Dwarka Subcity of Delhi
Weqar Ahmad Siddiqi1 and Javed Hasan1 *
1
Department of Applied Sciences and Humanities,
Faculty of Engineering and Technology,
Jamia Millia Islamia (Central University),
New Delhi,
India
DOI: http://dx.doi.org/10.12944/CWE.1.2.08
In the present study water samples, from various societies of East Zone of Dwarka subcity of New Delhi have been collected where rainwater-harvesting systems are installed. The impact of rainwater harvesting on the amounts of heavy metals in ground water has been studied by collecting water samples from these selected sites in the months before and after the rain. In study the difference between the values of some of the heavy metals in ground water, collected before and after rain, has been found. The present study reveals that the rainwater harvesting suitably reduced the amounts of heavy metals in under ground water and has improved the quality of ground water.
Copy the following to cite this article:
Siddiqi W.A, Hasan J. Effect of Rainwater Harvesting on the Amounts of Some Heavy Metals, in the Ground Water of East Dwarka Subcity of Delhi. Curr World Environ 2006;1(1):145-150 DOI:http://dx.doi.org/10.12944/CWE.1.2.08
Copy the following to cite this URL:
Siddiqi W.A, Hasan J. Effect of Rainwater Harvesting on the Amounts of Some Heavy Metals, in the Ground Water of East Dwarka Subcity of Delhi. Curr World Environ 2006;1(1):145-150. Available from: http://www.cwejournal.org/?p=607
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2006-11-28 |
|---|---|
| Accepted: | 2006-12-17 |
Introduction
Heavy metals are integral components of drinking water and have an important role in ascertaining the quality of drinking water for the drinking purpose. The amounts of the heavy metals should be adequately and as per the guidelines values provided by World Health Organization.
Among the heavy metals Iron is an essential element.1,2 It is contained in a number of biologically significant proteins, for example, hemoglobin and cytochromes, and also in many oxidation-reduction enzymes. The daily intake of iron from typical diets in developed countries has been estimated to be in the range of 15 to 22 mg.3,4,5 Zinc is an essential element for both animals and men and is necessary for the functioning of various enzyme systems, including alkaline phosphate, carbonic anhydrate, and alcohol dehydrogenate.6 Generally, Zinc concentrations in tap water vary between 0.01 and 1 mg/L.7
Chromium appears to be necessary for glucose and lipid metabolism and for utilization of amino acids in several systems. It also appears to be important in the prevention of mild diabetes and atherosclerosis in humans.8 Because of low solubility of chromium generally, the level found in water is usually low (9.7µg/L).9 Drinking water normally contains very low concentration of cadmium of the order of 1 µg/L or less10, 11-13; occasionally, levels up to 5 µg/L have been reported10 and on rare occasion levels up to 10 µg/L have been detected.14
The present study is related to the improvement in ground water quality using rainwater harvesting, wherein the physicochemical parameters have been determined to ascertain whether rainwater harvesting is useful for the ground water. The physicochemical parameters found improving the quality of ground water. Additionally, the studies on heavy metals were also conducted. During the study various samples, collected from the predetermined sites, were analyzed and amounts of heavy metals were determined. Subsequently, it was studied on the basis of determined values that how the rainwater harvesting is useful for the ground water quality.
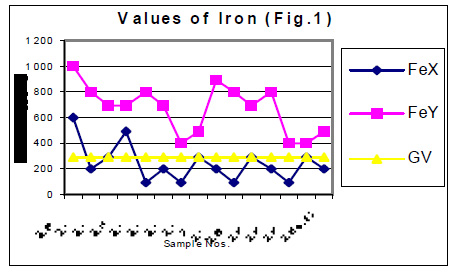 |
Figure 1 Click here to view figure |
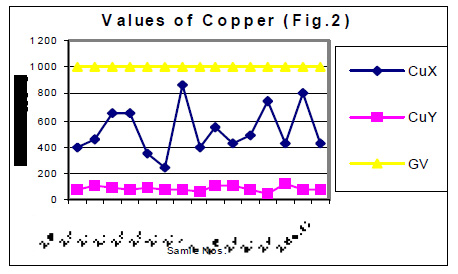 |
Figure 2 Click here to view figure |
Material and Methods
Reagents and Instruments
Atomic Absorption Spectrophotometer (AAS-300, FIAS) was used for determining the amounts of heavy metals in the below-mentioned water samples. Atomic Absorption Spectroscopy is one of the most widely used methods in analytical chemistry. This phenomenon can be divided into two major processes (i) The production of free atoms from the sample and (ii) the absorption of radiation from an external source by these atoms.
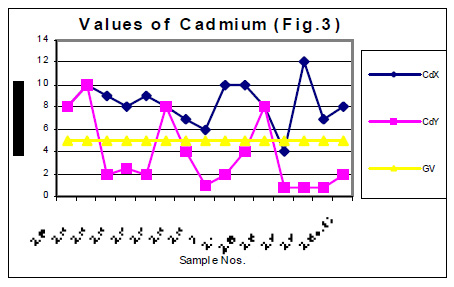 |
Figure 3 Click here to view figure |
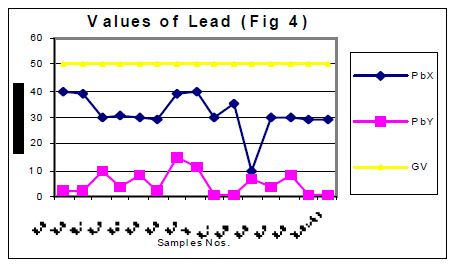 |
Figure 4 Click here to view figure |
The conversion of analyst in solution to free atoms in the flame is done by the flam atomizer. Here the metallic elements from its dissolved salts in the sample solution are converted into free atoms of the elements, by converting the sample solution into its aerosol. This aerosol when enters the flame, solvent is evaporated leaving small particles of the dry solid which is then converted into its vapor. Finally, this dissociates to give neutral free atoms. Some of these atoms are thermally excited by the flame but most remain in the ground state. These ground state atoms can absorb radiation of a particular wavelength that is produced by a special source made from that element. The wavelength of radiation given off by the source are the same as those absorbed by the atoms in the flame. The absorption follows Beers’ Law i.e. the absorbance is directly proportional to the path length in the flame and to the concentration of atomic vapour in the flame. However the path length may be held constant and the concentration of atomic vapour is directly proportional to the concentration of the analyte in the solution, which is displayed on electronic read out.
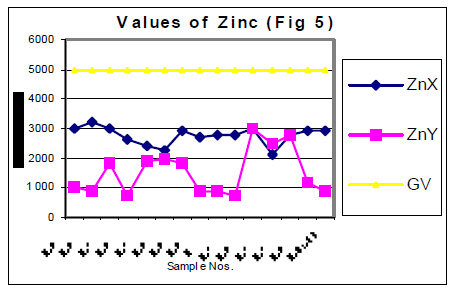 |
Figure 5 Click here to view figure |
Collection of Water Samples
Each fifteen ground water samples were collected on 1st day of July 2004 when it was almost no rain. The samples from the same sites were then again collected on 1st day of January 2005 after the rainy season. All samples were tightly sealed and immediately taken to the laboratory for analysis. During the analysis all samples were stored in dark and cool environment (at 4°C approx.)
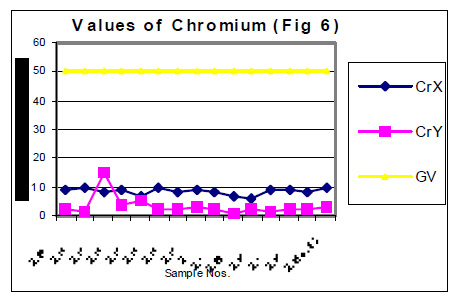 |
Figure 6 Click here to view figure |
Sampling Sites
Ground water samples collected from fifteen societies where rainwater-harvesting systems are installed. The names of the societies are as follows.
Sample No. Sampling Sites
-
Princess Park, Plot No. 33, Sector 6
-
Vidya sagar, Plot No.34, Sector 6
-
Surya, Plot No.14, Sector 6
-
Great India, Plot No.15, Sector 6
-
Akash Ganga, Plot No.17, Sector 6
-
Suruchi, Plot No.31, Sector 10
-
Shama, Plot No.32, Sector 10
-
Prabhavi, Plot No. 29, Sector 10
-
Man Bhavan, Plot No. 26, Sector 10
-
Rashi, Plot No.3, Sector 7
-
Shri Niketan, Plot No.1, Sector 7
-
HMM, Plot No. 6, Sector 10
-
Param Puneet, Plot No.27, Sector 6
-
Anusandhan, Plot No.22, Sector 6
-
RD., Plot No. 20, Sector 6
Data Analysis
The values of determined heavy metals i.e. Iron, Copper, Nickel, Cobalt, Cadmium, lead, Mercury, Zinc and Chromium have been mentioned in tables 1 and 2. Fig Nos. 1, 2, 3, 4, 5 and 6 show the graphical representation of the values of above mentioned heavy metals for the samples examined before and after monsoon. In the said figures X and Y specify the pre and post monsoon values and GV points out the guideline values prescribed by the World Health Organization. Additionally, Fig. -7 comprises the map depicting the study sites.
 |
Figure - 7(a): Map of Dwarka (Delhi) Click here to view figure |
Results and Discussion
Studies for determining some important heavy metals like Fe, Cu, Ni, Co, Cd, Pb, Hg, Zn and Cr in under ground water, collected before and after rainwater harvesting, performed using Atomic Absorption Spectroscopy and their amounts have been reported in (parts per billion) ppb. Using the same method, studies were also conducted for a sample of rainwater collected during the rainy season.
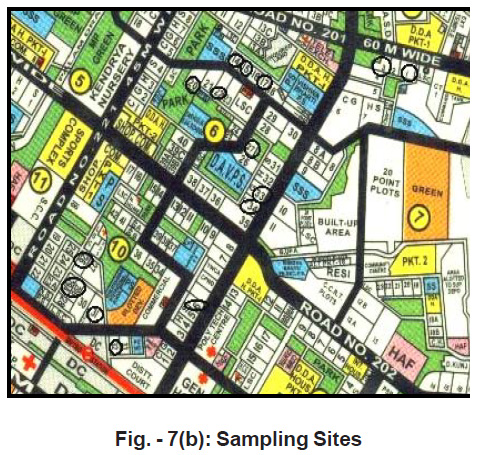 |
Figure - 7(b): Sampling Sites Click here to view figure |
Determined values (in ppb) for the water samples collected on July 1, 2004 before Rainwater Harvesting
| Sampling Nos. | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | R.W. | |
| Fe | 600 | 200 | 300 | 500 | 100 | 200 | 100 | 300 | 200 | 100 | 300 | 200 | 100 | 300 | 200 | 60 |
| Cu | 400 | 450 | 650 | 650 | 350 | 240 | 870 | 398 | 540 | 420 | 487 | 740 | 420 | 800 | 420 | 20 |
| Ni | - | 1 | - | - | - | - | 2 | 3 | - | - | - | - | 1 | - | - | - |
| Co | 1 | 1 | - | - | - | 2 | - | - | - | - | - | - | - | - | - | - |
| Cd | 6 | 10 | 5 | 10 | 7 | 11 | 5 | 5 | 4 | 3 | 6 | 7 | 4 | 8 | 6 | - |
| Pb | 40 | 39 | 30 | 31 | 30 | 29 | 39 | 40 | 30 | 35 | 10 | 30 | 30 | 29 | 29 | 3 |
| Hg | 1 | 0.9 | 0.8 | 2.5 | 4 | 8 | 0.9 | 2 | 4 | 5 | 4 | 3 | 8 | 0.8 | 4 | - |
| Zn | 3000 | 3200 | 3000 | 2600 | 2400 | 2300 | 2900 | 2700 | 2800 | 2800 | 3000 | 2100 | 2800 | 2900 | 2900 | 10 |
| Cr | 9 | 10 | 8 | 9 | 7 | 10 | 8 | 9 | 8 | 7 | 6 | 9 | 9 | 8 | 10 | - |
Determined values (in ppb) for the water samples collected on Jan1, 2005 after Rainwater Harvesting
| Fe | 1000 | 800 | 700 | 700 | 800 | 700 | 400 | 500 | 900 | 800 | 700 | 800 | 400 | 400 | 500 |
| Cu | 80 | 100 | 90 | 80 | 90 | 80 | 70 | 60 | 100 | 100 | 80 | 40 | 120 | 70 | 80 |
| Ni | - | - | 0.8 | 0.8 | 0.7 | 0.4 | 0.1 | - | - | 0.7 | 0.4 | 0.7 | 1 | 0.9 | - |
| Co | - | 0.4 | - | - | 0.8 | - | - | - | - | - | - | - | 0.2 | 0.1 | 0.4 |
| Cd | 8 | 10 | 2 | 2.5 | 2 | 8 | 4 | 0.9 | 2 | 4 | 8 | 0.8 | 0.7 | 0.8 | 2 |
| Pb | 2.2 | 1.9 | 10 | 4 | 8 | 1.9 | 15 | 11 | 0.8 | 0.7 | 7 | 4 | 8 | 0.8 | 0.7 |
| Hg | - | - | - | 1 | - | - | - | - | - | - | - | 1 | - | - | - |
| Zn | 1000 | 890 | 1800 | 750 | 1900 | 2000 | 1800 | 900 | 850 | 750 | 3000 | 2500 | 2800 | 1200 | 850 |
| Cr | 2 | 1.2 | 15 | 4 | 5.2 | 2.6 | 2.4 | 3 | 2.4 | 1 | 2 | 1.2 | 2 | 2.1 | 2.8 |
Note: S.No. & R.W. indicate the sample number and rain water respectively.
The amounts of Ni, Co, Cd, Hg, Zn and Cr in almost all the samples, collected in the month of July 2004 i.e. before rain, were found very low. These amounts are already distinctly below then their permissive limit in the water, which are shown in fig 4, 5 and 6. The amounts of these heavy metals were further decreased when similar studies were performed for the water samples collected in the month of January 2004 i.e. when the rainy season is over. Though the amount of cadmium in most of the samples (collected in July) were towards higher side than its permissible limits, they were also reduced due to rainwater harvesting. Results show that rain water collected during rain contain only little amounts of Fe (60 ppb), Cu (20 ppb), Pb (3ppb) and Zn (10ppb) which may be due to mixing of some environmental pollutants. An appreciable decrease in the amount of Cu and Zn has also been noticed for all the samples of under ground water as a result of harvesting due to rain. However a reverse trend was found with Fe, e.g. the amounts of Fe in the sample Nos. 1-5 (of July 2004) were found to be 600, 200, 300, 500 and 100 ppb. The amounts of Fe for the samples 1-5 (of January 2005) were sufficiently increased i.e. 1000, 800, 700, 700 and 800 ppb. This may be due to the solubility of some minerals present in the soil.
Conclusion
The studies, which have been performed on heavy metals for various samples of water collected before and after monsoon, show that the values of heavy metals for almost all the samples have been decreased appreciably due to the mixing of rain water to the under ground water. Thus these parameters indicate that rainwater harvesting suitably affects the quality of ground water. And rainwater harvesting has proved its utility for the above-mentioned sites.
Acknowledgements
Authors are thankful to Prof. Masood Alam, Head, Department of Applied Sciences and Humanities, for his kind cooperation and facilities in order to complete this work.
References
- Watt, B.K. and Merrill, A. L. Composition of foods – raw, Processed, prepared. Washington, D.C, Revised USDA Agriculture Handbook, (1963) 8.
- Moore, C. V. Iron. In: Good heart, R. S. and Shils, M. E. ed. Modern nutrition in health and disease. Philadelphia, Lea and Febiger, (1973) 297.
- Meranger, J. C. and Smith, D. C. The heavy metal content of a typical Canadian diet. Canadian Journal of Public Health, (1972) 63: 51.
- Bovering, J and Mac Pherson Sanchez, A. A conceptus of research on Iron requirements of man. Journal of Nutrition, (1976) 106: 985.
- Kirkpatrick, D. C. and Coffin, D. E. The trace metal content of representative Canadian diets in 1970 and 1971. Canadian Institute of food Science and Technology Journal, (1974) 7:56.
- Parisic, A.F. and Vallee, B.L. Zinc metalloenzymes : characteristics and significance in biology and medicine. American Journal of Clinical Nutrition, (1969) 22: 1222.
- Zoeteman, B.C. J. and Brinkman F.J. J. Human intake of minerals from drinking water in the European communities. In Hardness of drinking water and public health. Proceedings of the European Scientific Colloquium, Luxembourg, 1975. Oxford, Pergamon Press, (1976) 173.
- Towill, L. E. et al. Review of the Environmental Effects of Pollutants, III: Chromium. Cincinnati, US Department of Commerce, National Tech. Information Service, (1978) 282-796.
- National Research Council, Chromium. Washington, D. C, National Academy of Sciences, (1974).
- Commission of the European Communities. Criteria (dose/effects relationships) for cadmium, Oxford, Pergamon Press, (1978).
- Guidelines for Canadian drinking water quality, Quebee, supply and Services, 1979 (supporting documentation) (1978).
- Ambient water quality criteria for cadmium. Washington, D C, US Environmental Protection Agency, Office of WaterRegulations and Standards, Criteria and Standards Division, (1980).
- WHO Technical Report Series, No. 505, (Evolution of certain food additions and the contaminates: mercury, lead and cadmium) (1972).
- The hazards to health of persistent substances in water; annexes to a report of a WHO Working Group. Copenhagen, WHO Regional Office for Europe, (1972).
- Garg, V.K. and Totawat, K.L., Ground Water Contamination in the Area Adjoining Zinc Smelter Effluent Stream, Journal of Environ Science and Engg. (2004) 46(1), 61-64.
- Kaushik, A.K. ET AL. Heavy Metals Pollution of River Yamuna in the Industrially Developing state of Haryana. Indian J. Environ Hlth. (2001) 43(4), 164-168.






