Adaptive Leaf Structure and Anatomy in Rhizophora mucronata Lam.: The Effects of Salinity and Pollution on Foliar Characteristics
Swedha Madhavan Mavally1
*
 , Sreeja Pullaikodi2
, Sreeja Pullaikodi2
 , Silshalakshmanan Paledath2
, Silshalakshmanan Paledath2
 , Aiswarya Thekkayil1
, Aiswarya Thekkayil1
 and Chandramohanan Kotten Thazhath3
and Chandramohanan Kotten Thazhath3

1
Department of Botany,
Government Brennen College,
Kannur University,
Kerala
India
2
Department of Botany,
Sir Syed College,
Kannur University,
Kerala
India
3
Krishna Menon Memorial Government Women College,
Kannur University,
Kerala
India
Corresponding author Email: swedhamadhavan@gmail.com
Copy the following to cite this article:
Mavally S. M, Pullaikodi S, Paledath S, Thekkayil A, Thazhath C. K. Adaptive Leaf Structure and Anatomy in Rhizophora mucronata Lam.: The Effects of Salinity and Pollution on Foliar Characteristics. Curr World Environ 2025;20(1).
Copy the following to cite this URL:
Mavally S. M, Pullaikodi S, Paledath S, Thekkayil A, Thazhath C. K. Adaptive Leaf Structure and Anatomy in Rhizophora mucronata Lam.: The Effects of Salinity and Pollution on Foliar Characteristics. Curr World Environ 2025;20(1).
Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2025-02-10 |
|---|---|
| Accepted: | 2025-04-04 |
| Reviewed by: | 
 Nivedita Chaudhary
Nivedita Chaudhary
|
| Second Review by: |

 Yuva Raj
Yuva Raj
|
| Final Approval by: | Dr. Hiren B Soni |
Introduction
Mangroves are the only woody, facultative halophytes that grow at the ecotone between land and sea. Consequently, they acquired several morphological, physiological, biochemical, and molecular traits that support their ecological adaptation and persistence in extreme conditions, including fluctuating water levels and salinity.1
The anatomical features of mangrove species play two crucial roles: they serve as taxonomic identification tools3-6 and as an adaptation in response to fluctuating environmental stresses6,7such as elevated salt levels,8,9 pollution,10 sea level rise,11,12 inundation,13,14 varying light intensities,15 and high temperatures. Foliar adaptations of mangroves to various stresses include changes in the salt gland size and structure,16 size, and density of stomata,17 water storage tissues,18 cuticle thickening, leaf thickness, the thickness of palisade tissue,19 epidermal wax deposition,20 Casparian strip and suberin lamellae flexibility, and variations in vessel architecture.21,22 The plasticity of these structural alterations is a key element controlling mangrove survival and fitness. Research on the lamina characteristics of mangroves can help understand many aspects of plant life, including photosynthetic processes, production, sequestration of carbon dioxide, and stress tolerance.
Effective stress management is crucial for mangrove plant survival in the saline environment, leading to distinctive adaptations among species. Three types of true mangroves could be distinguished based on their resistance to salinities: species that are high, moderate, and less salt-tolerant. Rhizophora mucronata Lam. is a high salt-tolerant species but they don’t have salt glands like in the Avicennia species; they are salt excluders. Nevertheless, R. mucronata can survive in high salinity by preventing salt absorption at the root and excluding heavy salt through root ultrafiltration mechanism.32 Furthermore, R. mucronata accumulates salt in its older leaves, which are subsequently shed to remove the salt from the plant.33 Rhizophora mucronata trees were also found to increase their vessel density in response to high salinity, enabling improved water transport in hypersaline environments.34
R. mucronata is one of the dominant true mangrove species distributed in northern Kerala and also one of the major species recommended for planting in mangrove restoration programs. To ensure the conservation and restoration of the mangrove ecosystems, it is essential to comprehend how mangroves respond to regional and local changes.
Salinity surges and tides are important ecological constraints that limit the growth and survival of mangroves. Although most mangrove plants can withstand a saline level of 35 ppt, they flourish best in a range of 5 to 20 ppt.40-42 Water salinity in mangroves is influenced by both natural and anthropogenic activities. Precipitation, freshwater inflow, and sea level rise are major natural factors,43 and aquaculture, urbanization, land use changes, agriculture, and industrial effluent runoff are human-made factors influencing salinity and water quality.44 Anthropogenic activities lead to water pollution, which in turn alters the salinity.
This study aimed to examine the variation in leaf lamina characteristics of Rhizophora mucronata Lam. and investigate how it aids the species in thriving in a saline environment. Another objective is to determine whether variations in the water salinity and the water pollution in different sites selected from north Kerala impact the foliar properties of R. mucronata. The current investigation on environmental factors influencing the morphology and leaf lamina characteristics of Rhizophora species may give insight to improve management techniques and establish conservation plans at local sites, especially in areas where water pollution or salinity levels are increasing.
Materials and Methods
The genus Rhizophora includes seven species and three hybrids. All species of this pantropical genus are true mangroves, and their distribution is limited to the intertidal zone.24
Sampling sites
Mangroves cover an area of 19.531 km² in Kerala. Out of this, 50.05% is distributed in the northern districts of Kerala, including Kasaragod, Kozhikode, and Kannur.2
Seven different sampling sites (Table 1) were selected within the three northern districts of Kerala based on specific criteria such as population density and the distribution pattern of Rhizophora mucronata.
Sample leaf collection
R. mucronata plants with similar heights and diameters were chosen from each study site, and their mature leaves from the third node were taken away for foliar examination.45 Intact mature leaves were collected in the morning and immediately taken to the laboratory after being sealed in plastic bags.46
Table 1: latitude, longitude, and degree of pollution of selected study stations from north Kerala districts
Study stations | Latitude and longitude | Panchayat/ Village/ Municipality | Pollution |
C1: Kadalundi | N11º07.618' E075º49.969' | Kadalundi and Vallikunnu Village | Conserved area |
K1: Kumbala | N12º35.919' E074º56.626' | Kumbala Grama Panchayat | Medium Pollution |
K2: Mogral | N12º33.669' E074º57.360' | MogralPuthur Panchayat | High Pollution |
K3: Thalankara | N12º29.336' E074º59.235' | Kasaragod Municipality | Medium Pollution |
N1: Kunjimangalam | N12º05.646' E075º13.410' | Kunjimangalam Panchayat | Conserved area |
N2: Pazhayangadi | N12°01'37.7" E75°16'11.3" | Ezhome Panchayat | Medium Pollution |
N3: Valapatanam | N11º56.078' E075º21.075' | Valapatanam Grama Panchayat | High Pollution |
Morpho anatomical traits of leaf lamina
Laminar traits of R. mucronata studied include; Leaf area (LA) (cm²), Dry mass of leaf (DM) (g), Specific leaf area (SLA) = LA/DM (m²/Kg), Leaf mass per area (LMA) = 1/SLA (kg/m²), Lamina thickness (LTH) (µm), Leaf density (LD) = LMA/ Leaf thickness (LTH) (kg/m³), Leaf Fresh weight (FW) (g), Leaf Dry weight (DW), Moisture Content (MC%), mg Chlorophyll a/ g tissue, mg Chlorophyll b/ g tissue, Stomatal index (SI), Length of Stomata (SL) (µm), Width of Stomata (SW) (µm), Thickness of water storage/ non-assimilatory zone/colorless zone/ hypodermis (WST) (µm), Upper Palisade length (UPL) (µm), Lower spongy parenchyma thickness (SPT) (µm), UPL/SPT Ratio, Thickness of Upper Cuticle (UCT) (µm) and Thickness of Lower Cuticle (LCT) (µm).
Anatomic hand sections were taken at a point about halfway between the base and apex of the leaf lamina. The microscopic leaf anatomy slide preparations were analyzed using an Almicro Trinocular Microscope, and images were taken using a Magcam DC 5 microscope digital camera. Micrometric measurements were made using Magvision image analysis software.
Calculation of the stomatal index refers to research by Lestari (2006) using the formula that follows:
Stomatal index23 = number of stomata/ (number of stomata + number of epidermal cells) × 100
Chlorophyll estimation
Chlorophyll content was estimated following Arnon’s method (1949), with absorbance measured at 663 nm and 645 nm to determine chlorophyll a, chlorophyll b, and total chlorophyll content.
mg Chl. a/ g tissue = 12.7 (A663) - 2.69 (A 645) ×V ÷(100×W)
mg Chl. b/ g tissue = 22.9 (A663) - 4.68 (A 645) ×V ÷(100×W)
mg total Chl. / g tissue = 20.2 (A645) + 8.02 (A663) ×V ÷(10×W)
where W is the fresh weight (g) of the tissue extracted, V is the total volume (ml) of chlorophyll extracted in 80% acetone, and A663 and A645 are the absorbance at particular wavelengths (nm).
Analysis of the water sample was also conducted according to the APHA method (2017)31 to compare and study the effects of salinity and pollution on the foliar characters of R. mucronata. The parameters including pH (APHA, 2017 (Part 4500 H+)), Colour (APHA,2017 (Part 2120)), Hazen,30 Turbidity, NTU (APHA,2017 (Part 2130)), Total Dissolved Solids, mg/l (APHA,2017 (Part 2540)), Salinity, ppt (APHA,2017 (Part 2520B)), and Total Coliforms, CFU/100ml (APHA,2017 (Part 9222B)) from all the selected study sites were measured.
Table 2: Water quality parameters (mean ± standard deviation) measured at all sampling sites throughout the study period, including pH, Color (Hazen), Total Dissolved Solids (mg/L), Turbidity (NTU), Salinity (ppt), and Total Coliforms (CFU/100 mL).
Sl No. | Parameters | K1 | K2 | K3 | N1 | N2 | N3 | C1 |
1 | pH | 7.04±0.02 | 6.51±0.04 | 7.1±0.03 | 7.05±0.03 | 6.87±0.02 | 7.74±0.04 | 7.02±0.02 |
2 | Colour, Hazen | 15 | >20 | 10 | 5 | 15 | >20 | 15 |
3 | Total Dissolved Solids, mg/l | 13300±169 | 37204±235 | 17960±278 | 10030±306 | 15550±148 | 40328±107 | 21426±239 |
4 | Turbidity, NTU | 16.8±5.78 | 40.52±4.96 | 27.36±7.34 | 2.60±6.49 | 33.48±7.46 | 100.3±7.89 | 15.7±7.52 |
5 | Salinity, ppt | 29.42±3.46 | 36.05±2.06 | 26.93±2.47 | 18.37±3.44 | 25.21±2.51 | 38.42±3.29 | 30.27±2.48 |
6 | Total Coliforms, CFU/100ml | 1100 | 1100 | 400 | 400 | 1100 | 2400 | 400 |
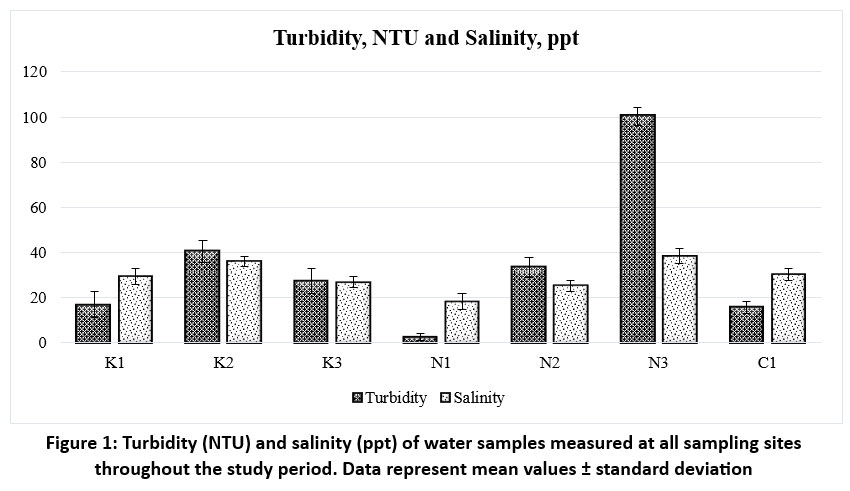 | Figure 1: Turbidity (NTU) and salinity (ppt) of water samples measured at all sampling sites throughout the study period. Data represent mean values ± standard deviation
|
Table 3: Laminar traits of Rhizophora mucronata at all sampling sites (mean ± standard deviation).
Laminar traits of R. mucronata | Study Sites | ||||||
K1 | K2 | K3 | N1 | N2 | N3 | C1 | |
LA (cm2) | 81.94±3.07 | 68.14±2.50 | 88.18±3.14 | 94.39±3.19 | 78.93±3.04 | 70.7±4.56 | 93.93±4.06 |
FW (g) | 4.08±0.04 | 6.79±0.06 | 5.39±0.07 | 3.86±0.09 | 4.36±0.11 | 6.28±0.13 | 4.49±0.21 |
DM (g) | 1.74±0.07 | 2.09±0.13 | 1.91±0.28 | 1.39±0.06 | 1.71±0.08 | 1.93±0.06 | 1.82±0.08 |
Moisture content % | 57.35 ± 0.02 | 69.20 ± 0.02 | 64.58 ± 0.05 | 64.04 ± 0.03 | 60.73± 0.03 | 69.32 ± 0.02 | 59.53 ± 0.05 |
SLA (m²/Kg) | 4.712±0.260 | 3.259±0.239 | 4.618±0.687 | 6.788±0.375 | 4.613±0.283 | 3.661±0.267 | 5.160±0.319 |
LMA (kg/m²) | 0.212±0.014 | 0.307±0.018 | 0.216±0.043 | 0.147±0.021 | 0.217±0.017 | 0.273±0.020 | 0.194±0.020 |
LD (kg/m³) | 304.79±20.08 | 415.96±24.33 | 330.0±65.79 | 230.47±32.89 | 295.94±23.13 | 360.06±26.38 | 300.03±30.96 |
0.819±0.012 | 0.438±0.021 | 0.766±0.005 | 0.983±0.014 | 0.794±0.025 | 0.379±0.017 | 0.913±0.024 | |
mg Chl. b/g tissue | 0.586±0.009 | 0.284±0.004 | 0.589±0.014 | 0.699±0.025 | 0.521±0.016 | 0.284±0.007 | 0.672±0.026 |
1.405±0.035 | 0.722±0.032 | 1.355±0.029 | 1.682±0.041 | 1.315±0.024 | 0.663±0.016 | 1.585±0.035 | |
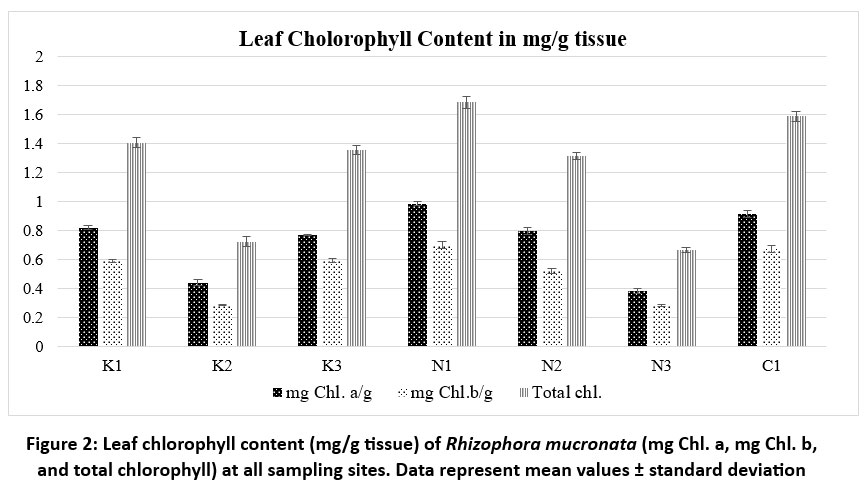 | Figure 2: Leaf chlorophyll content (mg/g tissue) of Rhizophora mucronata (mg Chl. a, mg Chl. b, and total chlorophyll) at all sampling sites. Data represent mean values ± standard deviation
|
The leaves of R. mucronata are simple, oppositely arranged,47dark green to yellowish green, shiny, hairless,47leathery, and crammed towards the tips of branches. Lamina was broadly elliptic or ovate-oblong and cuneated at the base.25 The margins are47smooth, with a pointed apex and a characteristic small needle-like tip. Midrib green beneath. The lower surface of the lamina has scattered black dots. These leaf-based structures, called cork warts (figure 5), help remove surplus salt from foliage and prevent it from accumulating in plant tissues. Cork warts form during leaf initiation and are present on the abaxial surface. They serve as an airway from the atmosphere to plant tissue, specifically aerenchyma cells.26,27
Table 4: Anatomical traits of Rhizophora mucronata leaf samples (mean ± SD) at all sampling sites.
Anatomical traits | Study sites | ||||||
K1 | K2 | K3 | N1 | N2 | N3 | C1 | |
WST (µm) | 275.52±3.49 | 281.43±4.08 | 279.92±2.47 | 242.54±2.94 | 274.01±3.09 | 294.74±3.78 | 259.07±2.69 |
UPL (µm) | 93.66±1.23 | 88.99±0.96 | 96.87±2.17 | 109.14±2.07 | 89.12±1.02 | 85.38±1.12 | 101.26±1.12 |
SPT (µm) | 287.09±0.92 | 300.55±1.24 | 270.35±1.26 | 276.02±1.13 | 295.89±1.01 | 354.40±2.01 | 286.77±1.35 |
UPL/SPT Ratio | 0.326 | 0.296 | 0.358 | 0.396 | 0.301 | 0.241 | 0.353 |
UCT (µm) | 0.77±0.01 | 1.12±0.03 | 0.67±0.02 | 0.62±0.02 | 0.85±0.01 | 1.23±0.03 | 0.63±0.01 |
LCT (µm) | 0.76±0.01 | 1.12±0.03 | 0.65±0.02 | 0.61±0.03 | 0.80±0.01 | 1.21±0.03 | 0.61±0.01 |
LTH (µm) | 696.26±1.25 | 738.94±0.94 | 653.03±1.34 | 638.5±1.19 | 734.81±1.28 | 756.26±1.87 | 645.43±1.02 |
SI | 7.45±0.12 | 6.34±0.11 | 8.22±0.14 | 8.73±0.11 | 7.26±0.18 | 6.47±0.11 | 8.54±0.18 |
SL (µm) | 11.23±0.73 | 14.91±2.05 | 10.88±0.36 | 10.47±0.0.81 | 13.77±0.81 | 15.26±0.44 | 11.45±0.54 |
SW (µm) | 8.08±0.04 | 9.02±0.08 | 8.14±0.08 | 7.45±0.05 | 8.23±0.06 | 9.22±0.084 | 7.14±0.05 |
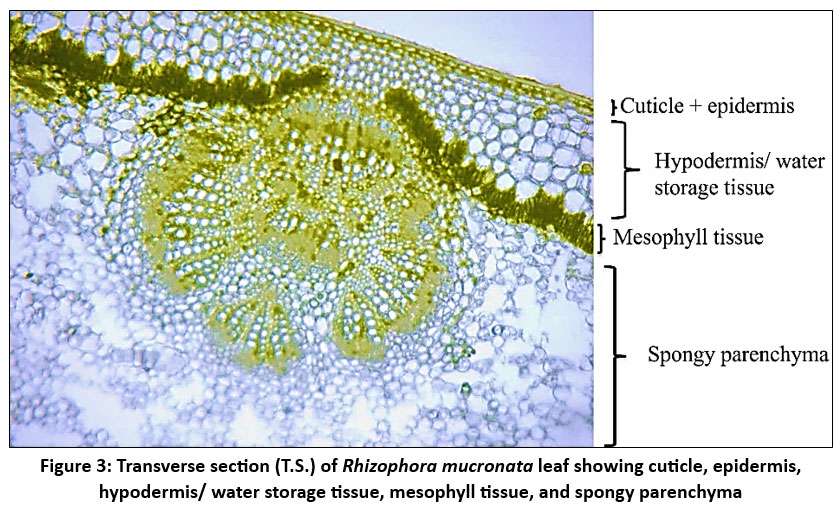 | Figure 3: Transverse section (T.S.) of Rhizophora mucronata leaf showing cuticle, epidermis, hypodermis/ water storage tissue, mesophyll tissue, and spongy parenchyma
|
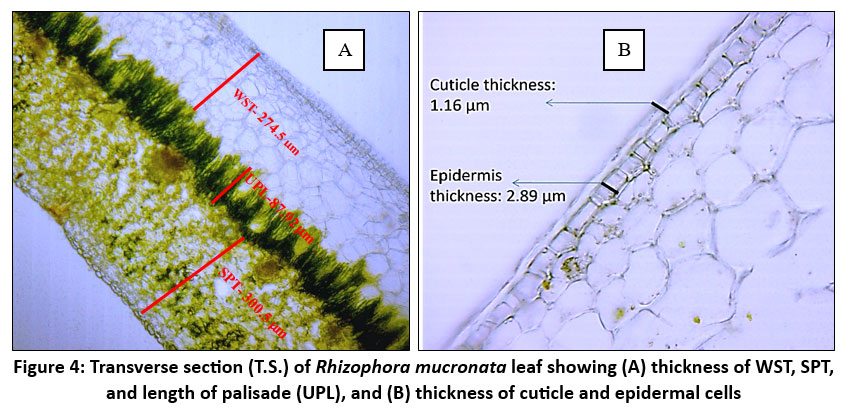 | Figure 4: Transverse section (T.S.) of Rhizophora mucronata leaf showing (A) thickness of WST, SPT, and length of palisade (UPL), and (B) thickness of cuticle and epidermal cells
|
 | Figure 5: Lower epidermal peel of Rhizophora mucronata leaf showing (A) stomata with length and width measurements and (B) cork warts
|
Discussion
R. mucronata distributed in sites like N3 (38.42 ppt) and K2 (36.05 ppt) are exposed to more saline water, which can result in the plants experiencing more osmotic stress. Compared to other low salinity sites like K1 (29.42±3.46 ppt), N1 (18.37±3.44 ppt), and N2 (25.21±2.51 ppt), thick cuticles, smaller leaf lamina, thick WST, and less dense, larger stomata are observed in leaves of R. mucronata at K2 and N3. Water analysis from all the sites also shows that the quality parameters like color, Hazen, total dissolved solids, mg/l, turbidity, NTU, and total Coliforms, CFU/100ml were also observed to be higher at the sites N3 and K2 compared to the conserved sites like N1 and C1. Water quality parameters (Mean±SD) across the study sites are given in table 2. Basyuni et al. assessed38Rhizophora mucronata growth during the first year of the restoration of mangroves at38abandoned ponds in Langkat, North Sumatra, and discovered that the landward zone was ideal for mangrove restoration using R. mucronata, with a 96% growth rate and a salinity concentration of 30 ppt.
According to a study by Hoppe-Speer et al., R. mucronata seedlings exhibit stress symptoms such as increased leaf necrosis when salinity rises, resulting in an overall decrease in growth.35The freshwater treatment (0 PSU) had the maximum stomatal conductance and photosynthetic performance, and the35lowest salinity treatment (8 PSU) showed the highest growth.35 The exact tolerance range of R. mucronata to salinity has not been fully explored because Aziz and Khan demonstrated that R. mucronata seedlings had optimal development at 17.5 PSU,35 while Jayatissa et al. discovered that R. mucronata seedlings were flourishing at 26 PSU. During the germination stage, R. mucronata exhibits a strong tolerance to salt. Low salinity greatly accelerated seedling growth, with 20.5 psu yielding the best growth. Furthermore, plant growth was negatively affected by greater salinities.39
The Hazen (1892) color, measures the intensity of the yellow color in a liquid on a scale from 0 to 500. High value of color Hazen in water indicates pollution and potential dissolved organic matter or contaminants. Water samples at K2 and N3 with a high Hazen color (>20) indicate higher levels of organic contamination. At K1, N2, and C1, the Hazen color (15) is moderate, indicating a moderate degree of organic pollution. At K3 (10) and N1 (5), low Hazen color (10 and below) indicates less pollution.
Turbidity, NTU also measures the water quality. Turbidity increases with the amount of total suspended solids in the water. Urban runoff, garbage discharge, and soil erosion can all contribute to turbidity.29 Anthropogenic activities might contribute to water pollution with significant suspended particulate matter, as indicated by a very high turbidity of more than 100 NTU at N3. Total Coliforms were also detected to be higher at N3 (2400 CFU/100ml). High levels of turbidity at K2 (40.52 NTU) and N2 (33.48 NTU) further indicate polluted conditions caused by industrial discharge, urban runoff, and waste disposal. At K3 (27.36 NTU) and K1 (16.8 NTU), moderate turbidity denotes moderate pollution, most likely from human or naturally occurring sources. N1 shows low turbidity (2.60 NTU) and comparatively less total Coliform count (400 CFU/100 ml), indicating comparatively less pollution.
In Rhizophora, the most prevalent characteristic is an increase in leaf thickness to cope with the saline environment.18 The thickness of the leaves increases when exposed to high saline conditions. To reduce the toxicity of salt to plant cells, high salinity induces increased leaf succulence.27 Bottom of FormThe range of mean LTH values, from 638.5 cm2 (N1) to 756.26 cm2 (N3), indicates that the LTH varies spatially. N3 exhibits the highest LTH (756.26 cm2±1.87), significantly thicker than all other samples, while N1 has the lowest LTH (638.5 cm2 ± 1.19). Table 4 shows the anatomical traits of R. mucronata leaf samples across the study sites.
R. mucronata leaf has a thick, multiseriate (2-6 layer) and well-developed hypodermis layer/ water storage tissue to retain water and shield the leaves from intense solar radiation. WST accommodates salt and water3,8 and aids in controlling salt levels in Rhizophora, especially when the plant sheds leaves in response to saline stress. The thickest WST (294.74 ± 3.78) was identified at site N3. At the sites, K2 and K3 also exhibit comparatively thick WST (281.43 ± 4.08 and 279.92 ± 2.47). Out of all the locations, C1 (259.07 ± 2.69) had the thinnest WST. Samples from the sites show relatively small standard deviations, suggesting that the thickness of WST is consistent within each site.
The hypodermis is followed by a compactly arranged palisade and spongy mesophyll cells. The deep placement of chloroplasts within the elongated palisade cells may reduce the impact of photodamage on the plant.18 Palisade cell length often correlates with the efficiency of the leaf to capture light and photosynthesize. The structure, shape, and size of palisade cells affect leaf photosynthesis.28 There are significant variations in palisade cell length between the study sites, particularly when comparing sites N1 and N3. The leaf UPL ranges from 85.38µm to 109.14µm among the sites. The longest palisade cells can be observed at N1 109.14 µm± 2.07, while the shortest ones are found at N3 85.38 µm± 1.12.
The low density of stomata and thick waxy cuticles are other important foliar characteristics observed in R. mucronata in high-saline sites. To minimize water loss through transpiration, leaves with thicker, waxy cuticles and fewer stomata are found. 27Larger stomata are more sparsely distributed on the surface of the leaf, allowing for better adaptation to saline conditions. The study by Ashraf et al. demonstrated that as salinity increased, Avicenna marina and Rhizophora mucronata considerably decreased their stomatal conductance.
The largest stomata are found at site N3, which has broader SW (9.22 µm) and longer SL (15.26 µm). The stomata of K2 are comparatively large, measuring 14.91 µm in length and 9.02 µm in width. N1 possesses the smallest stomata, measuring only 7.45 µm in width and 10.47 µm in length.
The leaf areas at seven study sites range from 68.14 cm2 (K2) to 94.39 cm2 (N1), with moderate variability within each site, as indicated by standard deviation values ranging from 2.50 to 4.56. Sites N1 (94.39±3.19), K3 (88.18±3.14), and C1 (93.93±4.06) show the largest average leaf areas, while Sites K2 (68.14±2.50) and N3 (70.7±4.56) have the smallest average leaf areas. Laminar traits, Chlorophyll a, Chlorophyll b, and total Chlorophyll content in mg/g tissue in the leaves of R. mucronata across the study sites were given in table 3.
The average range of the moisture content is 57.35% (K1) to 69.32% (N3). The two sites with the highest moisture content are K2 (69.20%) and N3 (69.32%). The lowest moisture content is found in K1 (57.35%). The intermediate moisture content readings at Sites K3, N1, N2, and C1 range from 59.53% to 64.58%.
The measurement of leaf surface area per unit of dry mass of leaf is called Specific Leaf Area/ (SLA). The highest SLA (6.788±0.375) was found in N1 and low SLA leaves at locations K2 (3.259±0.239) and N3 (3.661±0.267). Site K2 exhibits the highest leaf density (415.96 ± 24.33), which may be a sign of stress adaptation. The lowest leaf density can be observed in N1 (230.47 ± 32.89). The leaf densities are intermediate at K1, K3, N2, N3, and C1.
The total chlorophyll content (mg/g tissue) in R. mucronata leaf tissue was quantitatively assessed to be higher in K1 (1.405±0.035), N1 (1.682±0.041), and C1 (1.585±0.035). The leaves from locations K2 (0.722±0.032) and N3 (0.663±0.016) have relatively low levels of total chlorophyll. Other sites show relatively intermediate values (K3=1.355±0.029, N2= 1.315±0.024 and N3= 0.663±0.016). leaf chlorophyll content (mg/g tissue) of R. mucronata across the study sites is shown in figure 2. There is relatively little variation within each site, as indicated by the low standard deviation values, which range from 0.016 to 0.041 among the samples.
Conclusion
Significant foliar modifications such as low density of stomata, thick waxy cuticles, corky warts, thick water storage tissue/ hypodermis, and thick lamina were developed by R. mucronata to adapt to the stressful environment including water pollution and high salinity.
The laminar characteristics of R. mucronata, such as LA, LTH, WST, UPL, SI, stomatal size, and chlorophyll content, vary spatially and are influenced by water quality parameters like salinity, turbidity, color, pH, and TDS. Compared to the conserved sites like N1 and C1, sites N3 and K2 are identified as more polluted showing high water turbidity, total Coliform count, total dissolved solids, and high salinity.
Compared to less polluted and conserved sites like Kadalundi(C1) and Kunjimangalam(N1), R. mucronata distributed in more saline and polluted sites (K2 and N3) show variation in foliar characteristics as an adaptation and show low chlorophyll content in mg/g tissue, which indicates high salinity and water pollution impact the photosynthesis and productivity of Rhizophora. Therefore, immediate conservation measures must be implemented to preserve these polluted mangrove habitats.
Acknowledgement
The first, third, and fourth authors would likely acknowledge the Council of Scientific and Industrial Research (CSIR), Government of India, for providing the fellowship (SRF). We also express our special gratitude to the Principal, Government Brennen College, Dharmadam P O, Thalassery, Kannur, Kerala, 670 106, India, and the Kannur Kandal project, Edatt, Kunjimangalam field officers and team.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Permission to reproduce material from other sources
Not Applicable
Author Contributions
Swedha Madhavan M: Conceptualization, Methodology, Sample collection, Writing – Original Draft.
Sreeja P: Supervision, Review & Editing.
Silshalakshmanan P: Assist in field sample collection
Aiswarya T: Assist in field sample collection
Chandramohanan K T: Supervision, Review & Editing
References
- Friess D. A. Mangrove forests. Current Biology. 2016; 26(16):R746-R748. https://doi.org/10.1016/j.cub.2016.04.004
CrossRef - Pillai N. G., Harilal C. C. Inventory on the diversity and distribution of mangroves from the coastal ecosystems of Kerala State, India. Int J Recent Sci Res. 2018; 9(2):24002-24007. http://dx.doi.org/10.24327/ijrsr.2018.0902.1579
- Seshavatharam V., Srivalli M. Systematic leaf anatomy of some Indian mangroves. Proceedings: Plant Sciences. 1989; 99:557-565.
CrossRef - Surya S., Hari N. Comparative Study on Foliar and Petiole Anatomy of the Genus Bruguiera L. in Mangrove Forest of Kerala. Journal of Academia and Industrial Research. 2016; 5(7):92.
- Surya S., Hari N. Leaf and petiole anatomy of some members of Rhizophoracae (Mangroves) in Kerala. International Journal of Pharmaceutical Science and Research. 2017; 2(3):15-19.
- Tihurua E. F., Rahmawati K., Agustiani E. L., Ardhiyani M., Hutabarat P. W., Nasution T., ... & Junaedi D. I. Leaf anatomical characters of several true mangrove species. Berita Biologi. 2023; 22(1):111-128.
CrossRef - Rashid P., Shethi K. J., Ahmed A. Leaf anatomical adaptation of eighteen mangrove plant species from the Sundarbans in Bangladesh. Bangladesh Journal of Botany. 2020; 49(4):903-911. https://doi.org/10.3329/bjb.v49i4.52496
CrossRef - Putri R. Y., Bashri A. Anatomical Characteristics of Rhizophora’s Leaves as Mangrove Plant Adaptation at Banyuurip Mangrove Center. Jurnal Riset Biologi dan Aplikasinya. 2023; 5(2):98-109. https://doi.org/10.26740/jrba.v5n2.p98-109
CrossRef - Parida A. K., Das A. B., Mittra B. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees. 2004; 18:167-174.
CrossRef - Perez K. L. D., Quimado M. O., Maldia L. S., Tinio C. E., Hernandez J. O., Combalicer M. S. Effects of copper on the leaf morpho-anatomy of Rhizophora mucronata: Implications for mangrove ecosystem restoration. Biodiversitas Journal of Biological Diversity. 2021; 22(4). https://doi.org/10.13057/biodiv/d220454
CrossRef - Ellison A. M, Farnsworth E. J. Simulated sea level change alters anatomy, physiology, growth, and reproduction of red mangrove (Rhizophora mangle L.). Oecologia. 1997; 112:435-446. https://doi.org/10.1007/s004420050330.
CrossRef - Yanez-Espinosa L., Flores, J. A review of sea-level rise effect on mangrove forest species: anatomical and morphological modifications. Global warming impacts: case study on the economy, human health and on urban and natural environments. 2011; 253-276.
CrossRef - Wang W., Xiao Y., Chen L., Lin P. Leaf anatomical responses to periodical waterlogging in simulated semidiurnal tides in mangrove Bruguiera gymnorrhiza seedlings. Aquatic Botany. 2007; 86(3):223-228. https://doi.org/10.1016/j.aquabot.2006.10.003
CrossRef - Xiao Y., Jie Z., Wang M., Lin G., Wang W. Leaf and stem anatomical responses to periodical waterlogging in simulated tidal floods in mangrove Avicennia marina seedlings. Aquatic Botany. 2009; 91(3): 231-237. https://doi.org/10.1016/j.aquabot.2009.07.001
CrossRef - da Silva C. R. A., dos Santos Silva M., Ferreira L. M. D. S. L., Leite K. R. B., da Silva L. B. Morphological and anatomical aspects of the leaves of Rhizophora mangle L.(Rhizophoraceae) under different lighting conditions. Revista de Biologia Neotropical/Journal of Neotropical Biology. 2015; 12(2):74-80. https://doi.org/10.5216/rbn.v12i2.33405
CrossRef - Chi B. J, Guo Z. J, Wei M. Y, Song S. W, Zhong Y. H, Liu J. W, ... & Zheng H. L. Structural, developmental and functional analyses of leaf salt glands of mangrove recretohalophyte Aegiceras corniculatum. Tree Physiology. 2024; 44(1). https://doi.org/10.1093/treephys/tpad123
CrossRef - Qie Y. D., Zhang Q. W., McAdam S. A., Cao K. F. Stomatal dynamics are regulated by leaf hydraulic traits and guard cell anatomy in nine true mangrove species. Plant Diversity. 2024; 46(3):395-405. https://doi.org/10.1016/j.pld.2024.02.003
CrossRef - Ankure S, Tah M, Mondal S, Murmu A. K, Naskar S. Adaptive evolution of leaf anatomical features in mangrove Rhizophoraceae cues differential strategies of salt tolerance. Flora. 2023; 300:152225. https://doi.org/10.1016/j.flora.2023.152225
CrossRef - Zhang X. Y., Wee A. K. S., Kajita T., Cao K. F. Effects of provenance on leaf structure and function of two mangrove species: the genetic adaptation to temperature. Chinese Journal of Plant Ecology. 2021; 45:1241-1250.
CrossRef - Naskar S., Palit P. K. Anatomical and physiological adaptations of mangroves. Wetlands ecology and management. 2015; 23(3):357-370.
CrossRef - Surya S., Hari N. Ecological characterisation of true mangrove species in kerala. Environment. 2018; 11(12):13.
- Schmitz N., Jansen S., Verheyden A., Kairo J. G., Beeckman H., Koedam N. Comparative anatomy of intervessel pits in two mangrove species growing along a natural salinity gradient in Gazi Bay, Kenya. Annals of botany. 2007; 100(2):271-281. https://doi.org/10.1093/aob/mcm103
CrossRef - Lestari E. G. The relation between stomata index and drought resistant at rice somaclones of Gajahmungkur, Towuti, and IR 64. Biodiversitas Journal of Biological Diversity. 2006; 7(1). https://doi.org/10.13057/biodiv/d070112
CrossRef - Batool N. A. Z. I. M. A., Ilyas N. O. S. H. I. N., Shahzad A. R. M. G. H. A. N. Asiatic mangrove (Rhizophora mucronata)-An overview. European Academic Research. 2014; 2(3):3348-3363.
- Shamin-Shazwan K., Shahari R., Amri C. N. A. C., Kassim Z., Ahmad Z. Morphological structures of Rhizophora apiculata blume. and Rhizophora mucronata lam. Science Heritage Journal. 2021; 5(1):01-04. http://doi.org/10.26480/gws.01.2021.01.04
CrossRef - Evans L. S, Bromberg A. Characterization of cork warts and aerenchyma in leaves of Rhizophora mangle and Rhizophora racemosa. The Journal of the Torrey Botanical Society. 2010; 137(1):30-38.
CrossRef - Naskar S., Mondal S., Ankure S. Leaf anatomical adaptations of mangroves. Handbook of halophytes: From molecules to ecosystems towards biosaline agriculture. 2021; 1063-1077. https://doi.org/10.1007/978-3-030-57635-6_36
CrossRef - Gotoh E., Suetsugu N., Higa T., Matsushita T., Tsukaya H., Wada, M. Palisade cell shape affects the light-induced chloroplast movements and leaf photosynthesis. Scientific reports. 2018; 8(1):1472.
CrossRef - Patel H., Vashi R. T. Use of naturally prepared coagulants for the treatment of wastewater from dyeing mills. Characterization and Treatment of Textile Wastewater. 2015; 147-158. https://doi.org/10.1016/B978-0-12-802326-6.00006-X
CrossRef - Hazen A. A new color standard for natural waters. Chem. Jour.1892; 12:427-428.
- APHA. (2017). Standard Methods for examination of water and wastewater. In American Public Health Association (APHA).
- Ashraf M., Muhammad F., Hidayat J. W., Yaseen M., Ayyaz M., Ahmed W., Anwar M. H., Ahmed K. Comparison of salinity tolerance between Avicenna marina and Rhizophora mucronata Karachi coast, Pakistan. Journal of Bioresources and Environmental Sciences. 2023; 2(3): 89-99.
CrossRef - Steinke T. D. Mangroves in South African estuaries. Estuaries of South Africa. 1999; 119-140.
CrossRef - Schmitz N., Verheyden A., Beeckman H., Kairo J. G., Koedam N. Influence of a salinity gradient on the vessel characters of the mangrove species Rhizophora mucronata. Annals of Botany. 2006; 98(6): 1321-1330.
CrossRef - Hoppe-Speer S. C., Adams J. B., Rajkaran A., Bailey D. The response of the red mangrove Rhizophora mucronata Lam. to salinity and inundation in South Africa. Aquatic botany. 2011; 95(2): 71-76.
CrossRef - Aziz I., Khan M. A. Effect of Seawater on the Growth, Ion Content and Water Potential of Rhizophora mucronata Lam. Journal of Plant Research. 2011; 114(3): 369.
CrossRef - Jayatissa L. P., Wickramasinghe W. A. A. D. L., Dahdouh?Guebas F., Huxham M. Interspecific variations in responses of mangrove seedlings to two contrasting salinities. International Review of Hydrobiology. 2008; 93(6): 700-710.
CrossRef - Basyuni M., Telaumbanua T. F. C., Wati R., Sulistyono N., Putri L. A. P. Evaluation of Rhizophora mucronata growth at first-year mangrove restoration at abandoned ponds, Langkat, North Sumatra. In IOP conference series: earth and environmental science. 2018; 126: 1-5.
CrossRef - Patel N. T., Yadav D. R., Ghosh D., Pandey A. N. Salinity tolerance of Rhizophora mucronata Lam. from Gujarat coasts of India. Botanica Marina. 2010; 213-222.
CrossRef - Wang W., Yan Z., You S., Zhang Y., Chen L., Lin G. Mangroves: obligate or facultative halophytes? A review. Trees. 2011; 25: 953-963.
CrossRef - Patel N. T., Gupta A., Pandey A. N. Strong positive growth responses to salinity by Ceriops tagal, a commonly occurring mangrove of the Gujarat coast of India. AoB Plants. 2010; plq011.
CrossRef - Kodikara K. A. S., Jayatissa L. P., Huxham M., Dahdouh-Guebas F., Koedam N. The effects of salinity on growth and survival of mangrove seedlings changes with age. Acta Botanica Brasilica. 2017; 32(01): 37-46.
CrossRef - Wang W., Xin K., Chen Y., Chen Y., Jiang Z., Sheng N., Liao B., Xiong Y. Spatio-temporal variation of water salinity in mangroves revealed by continuous monitoring and its relationship to floristic diversity. Plant Diversity. 2024; 46(1): 134-143.
CrossRef - Bhagarathi L. K., DaSilva P. N. Impacts and implications of anthropogenic activities on mangrove forests: A review. Magna Scientia Advanced Research and Reviews. 2024; 11(1): 040-059.
CrossRef - Yuan G., Guo Q., Zhang Y., Gui Q., Xie N., Luo S. Geographical differences of leaf traits of the endangered plant Litsea coreana Levl. var. sinensis and its relationship with climate. Journal of Forestry Research. 2023; 34(1): 125-135.
CrossRef - Zhang S. B., Dai Y., Hao G. Y., Li J. W., Fu X. W., Zhang J. L. Differentiation of water-related traits in terrestrial and epiphytic Cymbidium species. Frontiers in Plant Science. 2015; 6: 260.
CrossRef - Taneo L. E., Areola M. B. Species account of mangroves in the coastal areas of Pangayawan, Gitagum, Misamis oriental, Philippines. Agricultural Science, Engineering and Technology Research. 2022; 5: 1-11.






