Effect of cell density and mutation on the expression of Rhi genes in Rhizobium leguminosarum biovar viciae
B. Boboye1 * and A. Downie2
1
Department of Microbiology,
Federal University of Technology,
Akure,
P. M. B. 704
Ondo State
Nigeria
2
John Innes Institute,
Centre,
Norwich,
NR4 7UH
Great Britain
DOI: http://dx.doi.org/10.12944/CWE.2.2.05
Effects of cell density, symbiotic plasmid and mutation (by transposon mutagenesis) on the expression of rhi genes in Rhizobium leguminosarum biovar viciae were studied. Strains of the bacterium bearing and lacking regulatory gene rhiR were grown to late exponential phase and assayed for the production of rhi genes inducer. R. leguminosarum biovar viciae was specific for the synthesis of the rhi genes inducer. Expression of rhi genes advanced with increased cell population without any difference between Sym minus and Sym plus plasmid strains of the bacterium. Highest amount of the inducer was produced in the exponential phase. The growth of the microbe with and without Sym plasmid showed that symbiotic plasmid enhanced the optimal formation of rhi genes inducer. Iniducer formation in the absence of Sym plasmid was less than 20 Miller units of b-galactosidase activity in contrast to 2375 Miller units for plasmid-containing strain. Tn-5 mutagenesis generated four groups of mutants. Classes I, II, III and IV mutants were low, moderate-, high- and super-producers of the inducer. These groups showed 145-250, 625-896, 1031-1375 and 1563 Miller units of phosphatase activity respectively.
Copy the following to cite this article:
Boboye B, Downie A. Effect of cell density and mutation on the expression of Rhi genes in Rhizobium leguminosarum biovar viciae. Curr World Environ 2007;2(2):135-140 DOI:http://dx.doi.org/10.12944/CWE.2.2.05
Copy the following to cite this URL:
Boboye B, Downie A. Effect of cell density and mutation on the expression of Rhi genes in Rhizobium leguminosarum biovar viciae. Curr World Environ 2007;2(2):135-140. Available from: http://www.cwejournal.org/?p=661
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2007-11-04 |
|---|---|
| Accepted: | 2007-12-11 |
Introduction
Rhizobium leguminosarum biovar viciae is a rod-shaped, motile, gram-negative, nitrogen-fixing bacterium which fixes nitrogen on legumes such as lentils, peas and vetches.1,2 These legumes like others are important in the provision of body-building minerals, calcium, vitamins A and B, thiamine, niacin and oil.3-7
Various research studies including works on rhi (rhizosphere-expressed) genes are being carried out on legume-rhizobia interaction to improve yields. These genes form an operon comprising of rhiABC. The operon is located adjacent to rhiR on symbiotic plasmid of R. leguminosarum biovar viciae.8 Rhizosphere-expressed genes are under the influence of rhiR. The rhiR is important in root nodulation. According to Gao et al.,9 the quorum-sensing system in a plant bacterium, Mesorhizobium huakuii affects growth rate and symbiotic nodulation. The rhi R encodes RhiR, a regulatory protein which functions as a transcriptional activator. RhiR has a homology with LuxR which belongs to LuxR-LuxI family. This class of transcriptional activators is common to a diverse group of gram-negative bacteria10,11 including the quorum-sensing signal molecules and the LuxRI homologs that were identified in fish pathogen Edwardsiella tarda.12 Comprehensive profiling of N-acylhomoserine lactones formed by Yersinia pseudotuberculosis using liquid chromatography coupled to hybrid quadrupole–linear ion trap mass spectrometry showed that the microbe belong to this group of transcriptional activators producers.13 Pseudomonas aeruginosa possesses LasR which functions to activate virulence. Agrobacterium tumefaciens synthesizes TraR which is responsible for conjugation control.14-17 Also, Agrobacterium vitis bears a luxR homolog, aviR, that is associated with induction of necrosis on Grape and a hypersensitive response on Tobacco.18 LuxR family protein SpnR which functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing is present in Serratia marcescens19. LuxR in Vibrio fischeri regulates the expression of an operon luxICDABEG in the presence of VAI (Vibrio autoinducer, N-3-(oxohexanonyl) homoserine lactone) thus influences luminescence in the microbe. LuxR-LuxI family is a population density-dependent gene activators.
The regulatory mechanism exhibited by LuxR-LuxI family is known as quorum sensing. It involves the interaction of self-produced extracellular signal compounds (autoinducers) with transcriptional activator proteins. In quorum sensing, after an adequate cell density is obtained, autoinducer normally accumulates which enables the bacterium to take a decision to regulate the expression of specific sets of genes10,20. Based on the homology which exists between RhiR and LuxR family, it was decided that investigation should be carried out on the principle of quorum sensing in R. leguminosarum bv. viciae. In order to do this, we studied the effects of cell density and mutation on the expression of rhi genes in the bacterium. This could give an indication to the regulatory circuits involved in the symbiotic properties of the R. leguminosarum bv. viciae.
Material and Methods
Organism and growth media
Rhizobium leguminosarum biovar viciae was obtained from American Type Culture Collection (Rockville, Md.). Rhizobium meliloti was kindly provided by Allan Downie with whom this work was carried out at John Innes Institute, Norwich, Great Britain. R. leguminosarum biovar viciae and R. meliloti lacking symbiotic (Sym) plasmids are these strains that have been cured of their Sym plasmids. They were grown in minimal medium21 and total yeast (TY) medium when required.²²
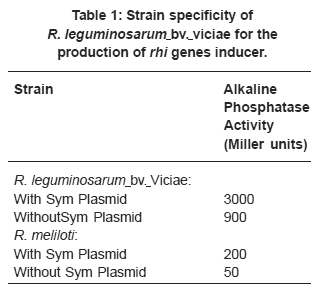 |
Table 1: Strain specificity of R. leguminosarum bv. viciae for the production of rhi genes inducer. Click here to view table |
Test for strain specificity for the production of rhi gene inducer.
Late exponential growth phase cultures of R. leguminosarum biovar viciae and R. meliloti with and without regulatory gene (rhiR) were tested for synthesis of the rhi gene inducer by alkaline phosphatase assay22 using a rhi C::phoA fused gene.
Test for the effect of cell density on rhi gene expression.
Strain of R.leguminosarum biovar viciae carrying rhiA::lacZ fused gene with and without symbiotic plasmid were grown at 280C in liquid TY medium,22 shaken at 180 rev min-1 for 72 hours. Cell growth by viable plate count method and β-galactosidase activity22 were determined during the incubation period at an interval of 6 hours.
Mutation experiment.
Transposon (Tn5) mutagenesis was carried out on mid-logarithmic phase cells of R. leguminosarum biovar viciae. One thousand one hundred and fifty mutants were screened on plate for the production of rhi gene inducer by alkaline phosphatase assay as described by22 Miller. Nineteen mutants found defective in the synthesis of the inducer were tested for alkaline phosphatase activity by liquid assay.22 Two controls (positive and negative) were prepared along with the mutants. The mutants were grouped into classes using respective 0-500, 501-1000, 1001-1500 and 1501- above Miller units of alkaline phosphatase.
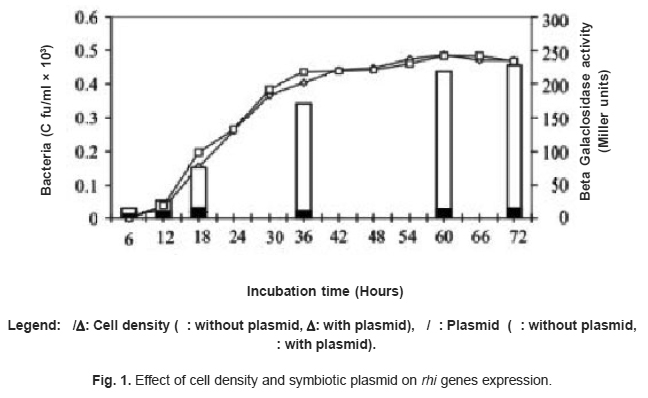 |
Figure 1. Effect of cell density and symbiotic plasmid on rhi genes expression. Click here to view figure |
Assay for β-galactosidase and alkaline phosphatase activities.
Methods described by22 Miller were employed. That of b-galactosidase was slightly modified and carried out as follows. Reaction mixture contained 0.9 ml culture and 0.2 ml isopropyl-thio-b-D-galactoside. The reaction was stopped after five minutes at room temperature (27oC) with 0.5 ml 1 M Na2Co3. Optical density of the mixture was read at 600nm and 420nm. The latter density was corrected to obtain the actual optical density. Units of b-galactosidase activity (Miller units) was defined as the absorbance at 420nm and 600nm per minute per millilitre of the reaction product under this assay condition.
Results
Phosphatase activity for synyhesis of rhiR inducer by R. meliloti was low relative to that of R. leguminosarum with and without symbiotic plasmid Table 1. Growth phase influenced rhi genes action Fig . 1. The colony forming units increased with incubation time in R. leguminosarum in the presence and absence of symbiotic plasmid. The two bacterial strains showed similar growth pattern without any significant difference. However, there was an increase in the activity of b-galactosidase with increased cell population in the presence of symbiotic plasmid with highest value of 237.5 Miller units in comparison with 12.5-17.5 Miller units for strain lacking symbiotic plasmid Fig. 1. This activity was highest in the exponential phase with pronounced increase in the mid-logarithmic phase than in any other growth phases.
Table 2 shows that the mutants were defective in the production of rhi genes inducer at various levels. Class I mutants (low-producers) having the inducer synthetic ability of 145-250 Miller units of phosphatase activity respectively comprised of mutant numbers 9, 15 and 16. Class II mutants (moderate-producers) were mutant numbers 1, 3, 4, 5, 6, 8, 11 and 14 with 625-896 Miller units. Class III mutants (high-producers, 1031-1375 Miller units) were made up of mutant numbers 2, 7, 10, 12, 13, 17 and 18 while class IV (super-producer) has a single member, number 19 showing formation ability of inducer at and 1563 Miller units. Relative to the negative and positive controls Table 2.
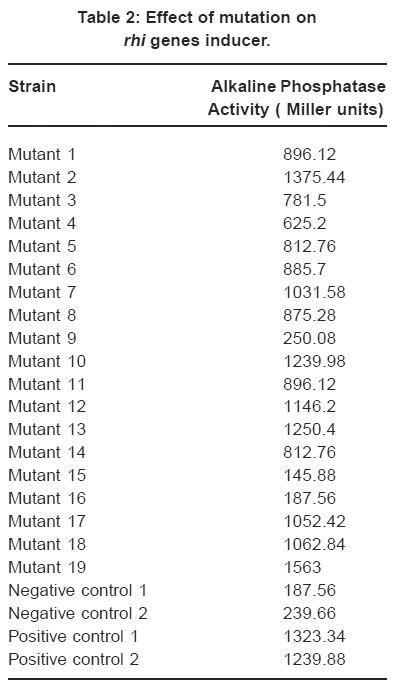 |
Table 2: Effect of mutation on rhi genes inducer. Click here to view table |
Discussion
Synthesis of large amount of rhi genes inducer by the R. leguminosarum than R. meliloti indicates that production of this metabolite is specific for the former bacterium and thus suggests that only certain rhizobial species form the compound. The inability of R. meliloti to express the rhi genes at high level was possibly due to presence of other genes located on the symbiotic plasmid which could block the production of appropriate metabolite to induce the rhi genes expression.
β-galactosidase activity highest in the exponential phase with pronounced increase in the mid-logarithmic phase than in any other growth phases indicates that rhi genes function optimally when the cells are in the active stage of their growth. Thus, the regulation of rhi genes by RhiR is growth phase and cell density dependent. Stationary phase cells appeared less active than logarithmic phase cells. Although, diversity in production of and response to N-acyl homoserine lactones is possible23; the result obtained here is similar to that reported by Fuqua et al.,10 and Gao et al.,11 Fuqua et al.,10 said that LuxR-LuxI family of transcriptional regulators are cell density dependent in Vibrio, Erwinia, Pseudomonas and Agrobacterium. Gao et al.,11 showed that rate of growth and symbiotic nodulation were directly related to the quorum-sensing system in Mesorhizobium huakuii. Many plant-associated bacteria regulate gene expression in a cell density-dependent manner by using quorum sensing via N-acyl homoserine lactones (AHLs). These AHLs pass out of and into bacterial cells. As the population of bacteria increases, so does the concentration of AHLs.24
The data on mutation implies that Class I mutants were strongly defective in rhi genes induction, class II were averagely defective and class IV mutant rhi genes were enhanced by the mutation to express the genes better than the wild type. Class III appeared unaffected being related to the wild-type strains (positive controls 1 and 2). This could be because rhi genes in this class were not affected by the Tn5 mutation to lack or produce the metabolite at lower or higher level in contrast to the last two Classes I and II.
Conclusion
Synthesis of rhi genes inducer was specific for R. leguminosarum biovar viciae. The bacterium expressed rhi genes in a cell density dependent pattern. Symbiotic plasmid is vital for the action of rhi genes; rhi genes induction is under the influence of rhiR which is located on the symbiotic plasmid. Tn-5 mutation affected the rhi genes induction.
Acknowledgements
The authors thank British Council, John Innes Institute, Great Britain and Federal University of Technology Akure, Nigeria for the financial support and instrumentation needed during this research work.
References
- Economou, A. and Downie, J. A., The nodulation of legumes by Rhizobia. In: The Nitrogen Fixation and its Research in China. Hong, D. (ed). Chapter 16: 316-333 (1992).
- Brewin, N. J., Ambrose, M. J. and Downie, J. A., Root nodules, Rhizobium and Nitrogen Fixation. In: Molecular Biology and Biotechnology. Casey, R. and. Davies, D. R. (eds). Peas Genetics, 237-289 (1993).
- Evans, H. J., Enhancing biological nitrogen fixation. Proceedings of a Workshop held on June 6, 1974. Sponsored by Energy Related General Research and the Division of Biological and Medical Sciences of the U. S. National Science Foundation Washington, D. C. (1975).
- Rachie, K. O., The nutritional role of grain legumes in lowland humid tropics. In: Biological Nitrogen Fixation in Farming Systems of the Tropics, Ayanaba, A., Dart, P. J. (ed). (1977) 45-61.
- F. A. O., Legume inoculation trial. In Technical Handbook on Symbiotic Nitrogen Fixation Rome: FAO of the United Nations, VI EXPE (1983) 1: 1/7-7/7.
- Singh, S. R., Impact of IITA’s grain legumes improvement program. In: Annual Report of International Institute of Tropical Agriculture, Ibadan, Nigeria. (1984) 5-33.
- Beijene, D., The state of research in Biological Nitrogen Fixation in Ethiopia. In: Biological Nitrogen Fixation in Africa. Proceedings of the last Conference of the African Association for Biological Nitrogen Fixation. Ssali, H. and Keya, S. O. (eds).(1985) 45-61.
- Cubo, M. T., Economou, A, Murphy, G., Johnston, A. W. B. and Downie, J. A., Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum. J Bacteriol., (1992) 174: 4026-4035.
- Gao, Y., Zhong, Z., Sun, K., Wang, H., and Zhu, J., The quorum-sensing system in a plant bacterium Mesorhizobium huakuii affects growth rate and symbiotic nodulation. Plant and Soil, (2006) 286: 1-2.
- Fuqua, W. C., Winans, S. C. and Greenberg, E. P., Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol., (1994) 176: 269-275.
- Swift, S. N., Bainton, J., Winson, M., K., Gram-negative bacterial communication by N-acyl homoserine lactones: a universal language?. Trends in Microbiol., (1994) 2: 193-198.
- Morohoshi, T., Inaba, T., Kato, N., Kanai, K. and Ikeda, T., Identification of quorum-sensing signal molecules and the LuxRI homologs in fish pathogen Edwardsiella tarda. J. Biosci. Bioeng., (2004) 98(4): 274.Ortori, C. A., Chhabra, S. A., Cámara, M., Williams, P. and Barrett, D. A., Comprehensive profiling of N-acylhomoserine lactones produced by Yersinia pseudotuberculosis using liquid chromatography coupled to hybrid quadrupole–linear ion trap mass spectrometry. Analytical and Bioanalytical Chemistry, (2007) 387(2): 497.
- Engebrecht, J., Nealson, K. and Silverman, M., Bacterial bioluminescence: isolation and genetic analysis of gene functions from Vibrio fischeri. Cell, (1983) 32: 773-781.
- Gambello, M. J. and Iglewski, B. H., Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol., 173, 3000-3009.
- Piper, K. R., Bodman, B. V. and Farrand, S.K., Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature (London), (1993) 362: 448-450.
- Fuqua, W. C. and Winans, S. C. A. LuxR-LuxI type regulatory system activates Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol., (1994) 176: 2796-2806.
- Zheng, D., Zhang, H., Carle, S., Hao, G., Holden, M. R. and Burr, T. J., A luxR Homolog, aviR, in Agrobacterium vitis is associated with induction of necrosis on grape and a hypersensitive response on Tobacco. Mol. Plant-Microbe Interactions,(2003) 16(7): 650.
- Horng, Y-T., Deng, S-C., Daykin, M., Soo, P-C., Wei, J-R., Luh, K-T., Ho, S-W., Swift, S., Lai, H-C. and Williams, P., The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens. Mol. Microbiol., (2002) 45(6): 1655-1671.
- Lithgow, J. K., Wilkinson, A., Hardman, A., Rodelas, B., Wisniewski-Dye, F., Williams, P., Downie, J. A., The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol., (2000) 37(1): 81-97.
- Jordan, D. C., Genus I. In: Bergey’s Manual of Systematic Bacteriology. Krieg N. R. and Holt, J. G. (eds), The William and Wilkind Co. Baltimore. (1984) 237.
- Miller, J.A., Experiments in Molecular Genetics. Cold Spring Harbor Laboratory, Press, Plainview, New York, (1976) 352-355.
- Jafra, S., Jalink, H., van der Schoor, R. and van der Wolf, J.M., Pectobacterium carotovorum subsp. carotovorum strains show diversity in production of and response to N-acyl homoserine lactones. J. Phytopathol., (2006) 154: 729–739.
- Winson, M. K., Camara, M., Latifi, A., Foglino, M., Chhabra, S. R., Daykin, M., Chapon, V., Bycroft, B. W., Salmond, G. P. C., Lazdunski, A., Stewart, G.S.A.B. and Williams, P., Multiple quorum sensing modulons interactively regulate virulence and secondary metabolism in Pseudomonas aeruginosa: identification of the signal molecules N-butanoyl-L-homoserine lactone and N-hexanoyl-L-homoserine lactone. Proc. Natl. Acad. Sci. USA., (1995) 92: 9427-9431.






