Pollution hazards in polyester dyeing and role of acid in monitoring
Namrata Kaushik1 , C.P. Kaushik1 , Sanjay Sharma2 * and J.K. Sharma1
1
Department of Chemistry,
Guru Jambheshwar University,
Hissar,
Haryana
India
2
Department of Chemistry and Environmental Engineering,
Institute of Engineering and Technology,
Alwar,
Rajasthan
India
DOI: http://dx.doi.org/10.12944/CWE.2.2.12
Environmental pollution is one of the greatest challenged for the world now days. The pollution load coming out from the number of sources like land, water and air pollution. By textile mill is mainly coming out the operation in wet processing. Present developments in Textile Wet Processing aim at over all clean environment. The Textile industry being highly water intensive is a major source of water pollution globally. Many dyes and chemicals used in textile processing remain unused after the absorption of dye in yarn or fabric. In this paper we discuss about the Disperse dyes, i.e. their absorption / exhaustion. Polyester dyeing with disperse dyes has been found to give much higher concentration of polluting substances resulting in greater harmful effects. All these chemicals are very hard to biodegrade and some are toxic too. We have studied other eco-friendly alternatives employing chemicals, which are less polluting and studied their effect on color strength (dye-absorption/exhaustion) and pollution load on different parameters in dye bath. Also discussed about dyeing the polyester dyeing with carrier method and their effect on color strength and comparison of pollution load on the different parameters in dye-bath.
Copy the following to cite this article:
Kaushik N, Kaushik C.P, Sharma S, Sharma J.K. Pollution hazards in polyester dyeing and role of acid in monitoring. Curr World Environ 2007;2(2):175-182 DOI:http://dx.doi.org/10.12944/CWE.2.2.12
Copy the following to cite this URL:
Kaushik N, Kaushik C.P, Sharma S, Sharma J.K. Pollution hazards in polyester dyeing and role of acid in monitoring. Curr World Environ 2007;2(2):175-182. Available from: http://www.cwejournal.org/?p=676
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2007-09-13 |
|---|---|
| Accepted: | 2007-11-07 |
Introduction
Dye stuffs give color to the material on which they are anchored. Dyeing is one of the most important operations in the Textile industry and a variety of dyes are used for dyeing (Telis 2001).
Dyes traditionally used for natural fiber coloration were water-soluble or solubilized prior to use and required salt linkages or vander Waals forces to impart affinity for the fiber. The dyes may be insolubilized within the natural fiber after being applied in a water soluble form, or they were made to react chemically with the fiber to prevent their removal. The development of disperse dye that is dye insoluble in water and applied from aqueous dispersion of synthetic fiber.
Polyester, a highly crystalline, thermopl-astic polymer, quite tightly packed together polyester is dyed with disperse dye. When dyed with disperse dyes has been found to give high concentration of polluting substances and results in greater harmful effects of waste water (Astray 2003). In this paper we discuss the dyeing of polyester by “High-temperature dyeing method” and “carrier method dyeing method”. Dyeing technology for the polyester fiber and fabric is complex but in comparison of the other dyes like vat, sulphur, direct dyes the disperse dyes gives more pollution load after the dyeing. The chief reason is the dispersing agent context in dyestuff, acetic acid, deformers, as well as the washing and reduction clearing process of dyeing. Disperse dyes are usually applied to polyester fiber in the pH range 4.5-5.5. It is important that the desired pH is maintained through the dyeing process to prevent fiber hydrolysis and dye degradation.
Acid is used in polyester dyeing to maintain an acidic pH (4.5-5.5) for which acetic acid is used. It is important that the desired pH is maintained through out the dyeing process. But due to the presence of two carbon atoms in one molecule of acetic acid its biodegradability is not ideal. So steps were taken to substitute acetic acid by other organic acids for maintaining the pH for dyeing efficiency at an optimum level. For the purpose of this study the two other acids used were oxalic acid and formic acid. These two acids are biodegradable and have low TOC (total organic carbon). Although oxalic acid has the same TOC as acetic acid, but it is a diacid, so the amount required is less. The TOC of formic acid is just half of acetic acid (During 88 and Paul 2003). The cause of pollution are unexhausted dyestuff and other chemical added to dye liquor as well as washing, cleaning process of dyeing which bring about high harmful effects (Deo et. Al. 9).
In polyester dyeing surface active agents are invariably added to assist in the dyeing process, i.e. additional dispersing agent may be used to help maintain dye in dispersion. Dispersing agents increase the solubility of the dye. In dyeing textured polyesters the introduction of an excess dispersing agent is inevitable for leveled shade .While the dyestuffs applied have 1/3 weight of a dispersing agent out their total weight. Some leveling agents, defoaming agents, wetting agents, and metal blocking agents may also be employed to increased rate of migration and leveling and fibre penetration (Smith and Mittal 99,Vidya, slack ). These dispersing agents made by the sodium salts of a cresol naphthalene sulphoric acid, formaldehyde condensate (Sampath 2003). Surface active agents are employed to prevent surface deposition of dye. Wetting agent, softner, soaps and detergents made by alkyl sulphates alkaryl sulphonate, ethylene oxide, fatty alcohol condensate,12 etc. All these chemicals are very hard to biodegrade and some are toxic too.13,14 After the dyeing process the unused dyes and chemicals cause greater effluent load and adverse effect to the environment.
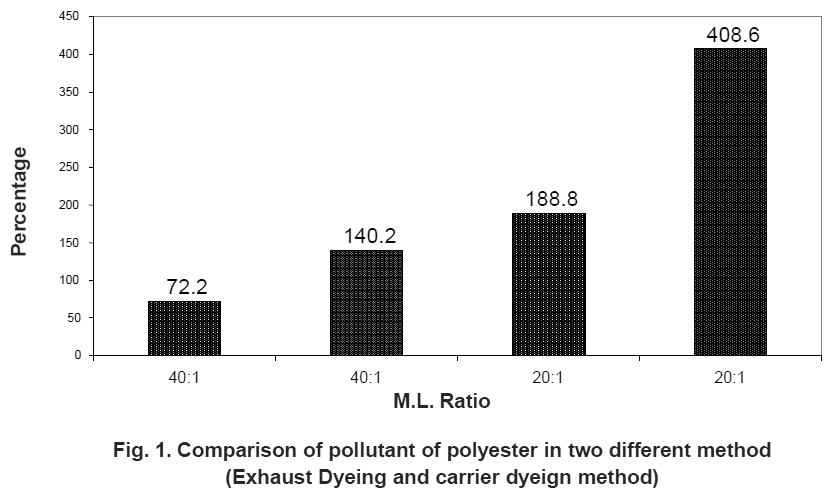 |
Figure 1. Comparison of pollutant of polyester in two different method (Exhaust Dyeing and carrier dyeign method) Click here to view figure |
Carriers applied in polyester dyeing most of the carriers contain emulsifying agent to facilitate dispersion on addition to warm water and are compatible with disperse dyes. Carriers are not fully absorbed by the fiber. The left part of carriers increases the waster water load. Carriers are volatile in steam or heat treatment also. In this reason causes air pollution. Carriers have offensive odour and some of the chlorinates types are significantly toxic to fish and bacteria sludge.15,16 Table 1 shows the pollution data for various carrier types.26,27
The first table compares the pollutant of different carriers but in polyester dyeing more harmful effect for environment. But the high-temperature dyeing method is less effluent or harmful effect as compares the carrier dyeing method. So carrier method should by replaced side by side. It shown in Table 2 and Fig. 1.
 |
Table 1: Comparison of pollution data for various carriers types Click here to view table |
In this paper we discuss comparison study of polyester dyeing with two different methods HT dyeing method and carrier dyeing and measured colour strength and pollution load in different dye bath. Also studied alternative of acetic acid pollution load on different parameters in dye bath.
Material and Methods
Dyeing
Dyeing of polyester component of P/V blended fabric with disperse dyes.
Machine
HTHP Glycerine Beaker Dyeing Machine, Computer Colour Matching System.
Fabric
Scoured polyester-viscose (65-35%) fabric used for this experiment Bleached and heat settled 200°C. The viscose part was removed by carbonization Dye/Shade: Disperse Dye.
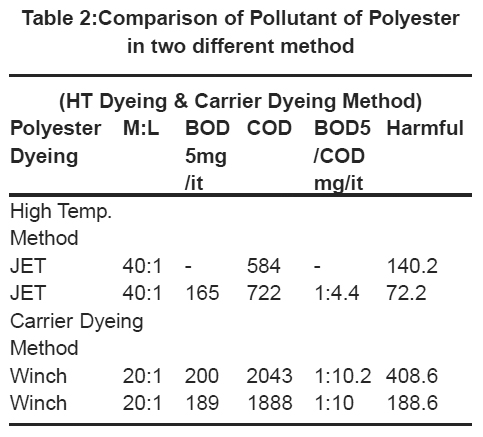 |
Table 2:Comparison of Pollutant of Polyester in two different method Click here to view table |
Acid
(30% strength) Acetic Acid, Formic Acid, Oxalic acid.
The following recipe was used:- Disperse dye - 1%, Wetting Agent - 1 gpl, Dyeing Assistants - 0.5 gpl, Acid - 0.5 gpl Dyeing temperature - 130°C, Time - One hour M.L. 1:20
|
Dye |
C.I. No. |
Structural formula |
|
Disperse Yellow |
42 |
10338 |
|
Disperse Red |
19 |
11130 |
|
Disperse Blue |
1 |
64500 |
Dyeing of Sample: (High-temperature Dyeing)
The requisite amount of dyes was pasteted and dissolved in water along with dispersing agent to make dye solution. Dyes solutions were prepared as per 1:20 M: L ratio at 4.5 to 5.5 pH was maintained by adding a drop of acid to each bath. The temperature was first raised to 60°C then 90°C and then 115°C at the rate of 1°C per minute. Temperature was further raised to 130°C. Dyeing was continued for about an hour at this temperature. Then the machine was cooled to 80°C by running cold water through outer jacket of the machine. The dyeing was carried out in dyeing tubes of laboratory dyeing machine (HTHP). All fabric sample were weighted 300 gram per each dyeing process 1/100 stock solutions of the dyes were prepared and used. The dye bath ratio for all dyeing sample was 1:20 M: L. Samples were taken out and preserved for unexhausted dye solutions were kept for further measurement of pollution load.
Dyeing of Sample (Carrier dyeing)
The dyeing of the samples was done in usual HTHP machine with carrier method, separately. In the carrier method only acetic acid was used. The temperature was 110+/5°C. The dyed sample and the exhaust dye baths were kept for the measurement of the dye uptake and effluent load respectively.
In the carrier method, carrier applied was 1gm/lt. Two carriers were used optional B (YCL), DilatinTCR (Sandoz).
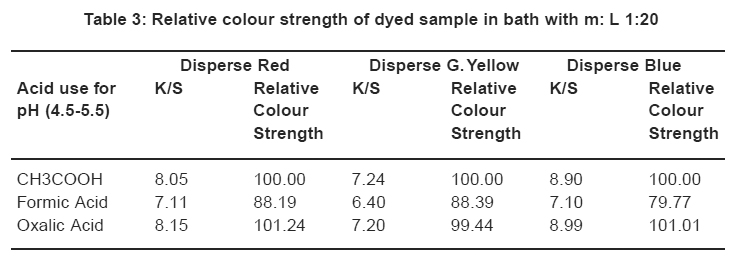 |
Table 3: Relative colour strength of dyed sample in bath with m: L 1:20 Click here to view table |
The dyeing of the sample done in the exhaust method and carrier method separately. The dyed sample and unexhausted dye baths were kept for measurement of dye uptake and effluent load respectively.
Measurement of K/S Value: All the K/s values were calculated from reflectance of the fabric at maximum absorption were length of the dyes. Over the visible range of 400 – 700 nm. The colour values were read from colour software was taken from 4 different parts of the fabric samples. The samples were folded 4 times for colour measurements.
Dyeing performance of various dyed samples was assessed by measuring the relative colour strength (K/S) by Perkin Elmer Computer Colour Matching System. These values are directly proportional to the concentration of colourant in the substrate. Its function gives relationship between reflectance and concentration.15
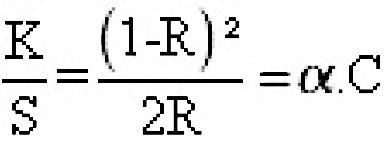
K = is the coefficient of absorption.
S = is the coefficient of Scattering.
a = is the optical constant at the wave length ë usually referred to as “Alpha value”.
Standard methods were used to determine the BOD (5 days), COD, D.S. and other user parameters.
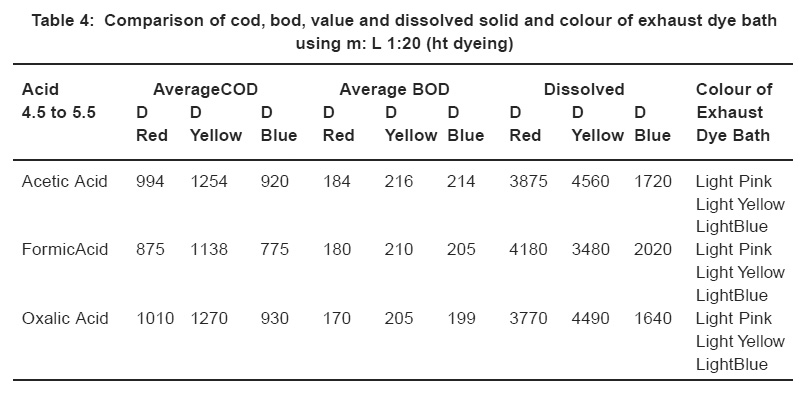 |
Table 4: Comparison of cod, bod, value and dissolved solid and colour of exhaust dye bath using m: L 1:20 (ht dyeing) Click here to view table |
Results and Discussion
HT Dyeing Method
Comparison in relative colour strength of the dyed samples (using three acids). The sample dyed with conventional method i.e. with acetic acid was considered as reference standard and it has been given colour pickup value of 100% as shown in Table 3.
Comparison of BOD, COD values, and dissolved solid and colour of exhausted dye baths Table 4.
Carrier Dyeing Method
Comparison of relative colour strength of dyed sample with disperse Red and two different carries. The sample dyed in the exhaust method with acetic acid considered 100% colour pickup value.
Comparison of COD value and dissolved solids.
Table 3 shows the relative colour strength of samples dyed with Disperse Red, Disperse Blue and Disperse Yellow using acids for controlling pH. For comparison the samples dyed in the presence of acetic acid were taken as Reference Standard and the value of colour strength for the same is taken as 100%. In comparison with the relative colour strength of samples dyed in the presence of Oxalic acid comes to 101.24, 99.44, and 101.01. From these results it can be surmised that Oxalic acid gives higher colour strength in comparison to formic acid and also acetic acid.
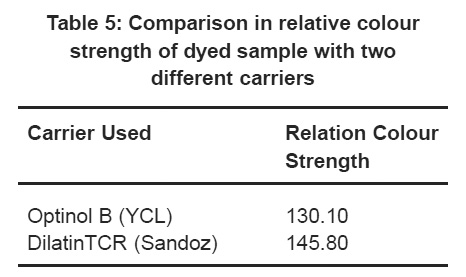 |
Table 5: Comparison in relative colour strength of dyed sample with two different carriers Click here to view table |
It is seen from Table 4 that with the use of Formic acid the COD and BOD values are the lowest. With Oxalic acid the BOD and Dissolved Solid Values range from 170 to 205 mg/lt. and 1640 to 4490 ppm, but the COD values have slightly increased. In the case of conventional method (HT dyeing method) the BOD and Dissolved Solid Values range from 184 to 216 mg/lt. and 1720 to 4560 ppm. It can, therefore, be concluded that with the use of Oxalic acid the rate of exhaustion in Disperse Dyes is maximum along with minimum or less effluent load.
It is seen from Table 5 with the relative colour strength of dyed samples with disperse Red 19 and two different carriers. The sample dyed with acetic acid was considered having 100% colour pick value.
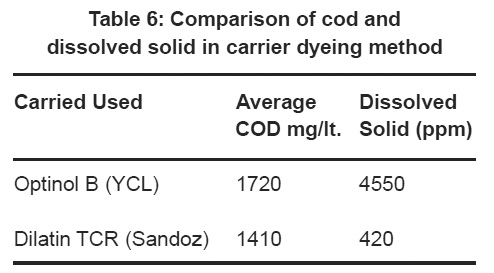 |
Table 6: Comparison of cod and dissolved solid in carrier dyeing method Click here to view table |
Table 6 shows the comparison in COD values and dissolved solids. lt has earlier mentioned that the use of the carrier produce more pollution load in effluent confirmed here by the result. By the carrier dyeing method the colour picks up value by the fabric is quite satisfactory but the COD value and dissolved solids value increased in optional (YCL). But the other carrier the Dilatin TCR provides better result .Carriers having hydroxyl groups on their benzoid structure. Which are hard to degrade. So the application of carrier must be less or restricted is polyester dyeing. So for the better environment or green environment must to used the exhaust dyeing method.
Conclusion
Polyester is invariably dyed with disperse dyes which give high concentration of polluting substances. In the conventional method the problem is further aggravated by using acetic acid for maintaining an acidic pH. Other organic acids have been found to give low pollution load. Of these the oxalic acid gave maximum dye uptake and better exhaustion of dye bath with minimum pollution load. Oxalic acid being a diacid causes less pollution load since the amount of acid required is less. Oxalic acid is followed by acetic acid. Formic acid results in reduced colour pickup. In all the three acids the BOD and DS are approximately the same, but there is slight increase in COD with oxalic acid. Although formic acid reduces the pollution load, but the colour strength is also slightly lower. The dye operation can be optimized at the colour strength value at the level of conventional method and minimizing the pollution load by judicious use of oxalic acid in the recipe. The dosing of oxalic acid needs to be strictly monitored throughout the process. In second polyester dyeing method (carrier dyeing) the harmful factor COD and dissolved solid and BOD are higher. The BOD and dissolved solids in both methods are higher. Carriers are toxic in nature and cause air pollution. The other limitation of this method is reduced light fastness and spot effect. Comparison of both methods HT Dyeing is better for dyeing the polyester.
References
-
Teli et. al, Processing of Sulphur and Vat Dyes. I-J-FandT, March-June (2001) 01-102.
-
Gandhi R.S., Chemical Processing of Synthetics and Blends-Impact Environment and Solution. Indian Journal of Fibre and Textile Research, (2001) 125-135.
-
V.A. Shenai-vol (2), Chemistry of Dyes and Principles of Dyeing, Sevak Publications, (285-331)
- Disperse Dye.htm on wikipedia org/wiki/ polyester
- Asrey Ram, Textile Processing in India and Impact on Surface Water Quality. Colourage, Oct, (2003) 23-25
-
Deo et. al., Green Tech. in Textile Processing of P/V, I.J.F and T. (1999) 24: 64-69.
-
Durig, Textileveredlung, (1976) 11: 62.
-
Paul S.K. Chaudhary, The Latest Dyeing Tech. of Polyester Sewing Thread. Colourage, March (2003) 56-58.
-
Smith. Brent, Pollutant Source Reduction (Part I and II), American Dye Stuff, March, (1999) 28-30.
-
Mittal R.M. Trivedi, S.S, Chemical Processing of Polyester Cellulose Blends, ATIRA DIGEST, (1983) 82-85.
-
Sampath M.R., Pollution Abatement, Part – II. Colourage, January, (2003) 76-80
-
Prof. Achwal W.B., Textile Softners. Colourage, March, (2002) 69-82.
-
Shah K.M. – Hand Book of Synthetics Dyes and Pigments, 131-156
-
Waring and Halls, The Chemistry and Application for Dye, Plenum Press, (107-161)
-
Becere, Behcet, An Approach for Estimation of Relationship Between K/S Value and Dye Uptake. Colourage, May (2003) 39-48.
-
Allen, E, ‘Optical Radiation Measurements Vol. 2 colour measurement, Ed. S F. Grum of C.J. Bartleson, Academic Press, New York, Chapter 7 Colorant formulation and shading, (1980) 303-305.
-
Brockes, Dr. A, Strocka, Dr. D., Berer Schunj Dr. A., Colour Measurement in the Textile Industry, Bayer, 27-29
-
Etters J. N. American Dyestuff Reporter, (1997) 80: 15-16.
-
McDonald R ‘Colour physics for Industry,’ SDC, Bradford, Chapter 5, (1987) 117-121.
-
Maharen, K, The Colour Science of Dyes of pigments, Adam Hilger Ltd., Brirtol, 2nd Edition, Chapter (1986) 12: 183.
-
Deo Wasif Desai, Charaborty Green Technology in textile processing. Indian Journals Fiber and Textile Research. (1999) 24: 64-69.
-
Allen, E, Optical Radiation Measurements Vol. 2 Colour Measurement, Ed. S. F. Frun and C. J. Bartleson, Academic Press, New York, Chapter 7 Colorant Formulation and shading, (1980) 303-305.
-
Brockes, Dr. A, Strocka, Dr. D, Berger – Schun, Dr. A Colour Measurement in Textile Industry, Bayer 27, 29.
-
Etters, J. N., Textile Chemist and Colorist. (1990) 22(6): 29-30.
-
Etters J. N. American Dyestuff Reporter, (1997) 86(3): P-15-17. edilck P, et al, Mellianel Text. (1970) 57: 583-587. aidhya, A.A, Trivedi S. S. Textile Auxiliaries and Finishing Chemicals ATIRA p-35 (1975).
- Richer, Text levered (1976) 11: 62.
- During, Text levered lung, (1976) 11: 46.
-
Vaidya A. A. Colour Chronicla A. Sandoz Publication Jan-March (1984).
-
Slack I. Can Text, Jr July 46 (1979).
-
Carbonell, J. ADR, (1962) Jan 5: 17(83).
-
S. M. Burkinshaw, The Chemistry of Application of Dyes Plenum Publication P-203 aidhya, A.A, Trivedi S. S. Textile Auxiliaries and Finishing Chemicals ATIRA p-35 (1975).
-
Allen, E, ‘Optical Radiation Measurements Vol. 2 colour measurement, Ed. S F. Grum of C.J. Bartleson, Academic Press, New York, Chapter 7 Colorant formulation and shading, (1980) 303-305.
-
Allen, E, Optical Radiation Measurements Vol. 2 Colour Measurement, Ed. S. F. Frun and C. J. Bartleson, Academic Press, New York, Chapter 7 Colorant Formulation and shading, (1980) 303-305.
-
Asrey Ram, Textile Processing in India and Impact on Surface Water Quality. Colourage, Oct, (2003) 23-25.
-
Becere, Behcet, An Approach for Estimation of Relationship Between K/S Value and Dye Uptake. Colourage, May, (2003) 39-48.
-
Brockes, Dr. A, Strocka, Dr. D, Berger- Schun, Dr. A Colour Measurement in Textile Industry, Bayer, 27, 29
-
Brockes, Dr. A, Strocka, Dr. D., Berer Schunj Dr. A., Colour Measurement in the Textile Industry, Bayer, 27-29
-
Carbonell, J. ADR, Jan 5, (1962) 17(83) .
-
Deo et. al., Green Tech. in Textile Processing of P/V, I.J.F and T. March, (1999) 24: 64-69.
-
Deo Wasif Desai, Charaborty Green Technology in textile processing. Indian Journals Fiber and Textile Research (1999) 24: 64-69.
-
Disperse Dye.htm on wikipedia org/wiki/ polyester
-
Durig, Textileveredlung, (1976) 11: 62.
-
During, Textileveredlung, (1976) 11: 46. edilck P, et al, Mellianel Text. (1970) 57: 583-587.
-
Etters J. N., American Dyestuff Reporter, (1997) 80: 15-16.
-
Etters J. N. American Dyestuff Reporter, (1997) 86(3): 15-17.
-
Etters, J. N., Textile Chemist and Colorist (1990) 22(6): 29-30.
-
Gandhi R.S., Chemical Processing of Synthetics and Blends – Impact Environment and Solution. Indian Journal of Fibre and Textile Research, March – June, (2001) 125-135.
-
Maharen, K, The Colour Science of Dyes of pigments, Adam Hilger Ltd., Brirtol, 2nd Edition, Chapter (1986 ) 12: 183.
-
McDonald R ‘Colour physics for Industry,’ SDC, Bradford, Chapter 5, (1987) 117-121.
-
Mittal R.M. Trivedi, S.S, Chemical Processing of Polyester Cellulose Blends, ATIRA DIGEST, (1983) p-82-85
-
Paul S.K. Chaudhary, The Latest Dyeing Tech. of Polyester Sewing Thread. Colourage, March, (2003) p 56-58.
- Prof. Achwal W.B., Textile Softners. Colourage, March, 69-82 (2002)
- Richer, Text levered (1976) 11: 62.
- S. M. Burkinshaw, The Chemistry of Application of Dyes Plenum Publication P-203.
- Sampath M.R., Pollution Abatement, Part – II. Colourage, January, (2003) 76-80.
- Shah K.M. – Hand Book of Synthetics. Dyes and Pigments, (131 – 156)
- Slack I. Can Text, Jr July, 46 (1979).
- Smith. Brent, Pollutant Source Reduction (Part I and II), American Dye Stuff, March, (1999) 28-30.
- Teli et. al, Processing of Sulphur and Vat Dyes. I-J-FandT, March-June, (2001) 1-102.
- V.A. Shenai – vol (2), Chemistry of Dyes and Principles of Dyeing, Sevak Publications, p 285-331.
- Vaidya A. A. Colour Chronicla A. Sandoz Publication Jan-March (1984).
- Waring and Halls, The Chemistry and Application for Dye, Plenum Press, 107-161.






