Assessment of physico-chemical parameters of drinking water of Bhitarwar town, Gwalior, M.P. India
Naveen Kumar Singh1 * , K.P.S. Chauhan2 and D. S. Kadam3
1
Chemical Research Laboratory,
SMS Government Model Science College,
Gwalior,
India
2
Dr. B.S. Govt. P.G. College,
Gwalior,
India
3
Government Chemical Laboratory,
Div. Ground water Survey Unit-2,
Gwalior,
India
DOI: http://dx.doi.org/10.12944/CWE.3.1.23
Safe drinking is the primary need of every human being .Some ions dissolved in water are essential for human beings, if present in appropriate concentration, while higher concentration of the same can cause adverse effect to human health. Ten(10) water samples are collected from Bhitarwar Town and Near by Villages(Gwalior District).Some parameter are within the permissible limits as prescribed by ISI and W.H.O. while other is beyond the limits. The ionic concentration is expressed in mg/L.
Copy the following to cite this article:
Singh N.K, Chauhan K.P.S, Kadam D.S. Assessment of physico-chemical parameters of drinking water of Bhitarwar town, Gwalior, M.P. India. Curr World Environ 2008;3(1):153-156 DOI:http://dx.doi.org/10.12944/CWE.3.1.23
Copy the following to cite this URL:
Singh N.K, Chauhan K.P.S, Kadam D.S. Assessment of physico-chemical parameters of drinking water of Bhitarwar town, Gwalior, M.P. India. Curr World Environ 2008;3(1):153-156. Available from: http://www.cwejournal.org/?p=791
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-04-25 |
|---|---|
| Accepted: | 2008-05-30 |
Introduction
Water, after air is the second most essential and vital component for the survival of all types of living beings. Water, like air and food is a basic human need without which no life can be sustained. The surface water resources catered for a long time a substantial amount of requirements for domestic, agricultural and industrial purposes. However, the growing population explosion, the rapid industrial growth and extensive cultivation of large areas have created a shortage of economically usable surface water resources. Ground water is a precious renewable resource which gets replenishment from the precipitation. The total ground water demand is likely to grow substantially from 3000 Km3 in 1990 to 4300 Km3 in 2050.1 The Physical and chemical quality of ground water is affected by industrial activities and human activities. Ground water is the most important source of water supply for irrigation, Industries and for drinking purposes. All ground water sources are not always safe. The present paper deals with the drinking water quality analysis of Some Hand pumps and well of Bhitarwar town and near by villages (Gwalior,M.P.) .The results were compared with W.H.O. and I.S.I. standard.
Material and Methods
For the analysis of physico-chemical parameters in water Ten (10) samples were collected in wide –mouth plastic bottles. pH values of the ground water samples under investigation were measured using systronic pH meter, type 361.The pH meter standardized by buffer solution of 4.0pH and 9.2pH .Total Alkalinity of the ground water samples were determined by titrating With N/50 H2SO4 using phenolphthalein and methyl orange as an indicator.
The total hardness of the water samples were determined by complexometric titration with EDTA using eriochrome black-T as an indicator. The calcium hardness of the water samples were determined by complexometric titration with EDTA using ammonium parpurate as an indicator. The estimation of chloride ions is generally determined by titrating the water sample against a standard solution of silver nitrate using potassium chromate as an indicator. Sodium and potassium were estimated using flame photometer (128) technique. NO32-,SO42- were estimated using UV-visible spectrophotometer. EC values of the ground water samples under investigation were measured using systronic EC meter. T.D.S. is measured by gravimetric method.
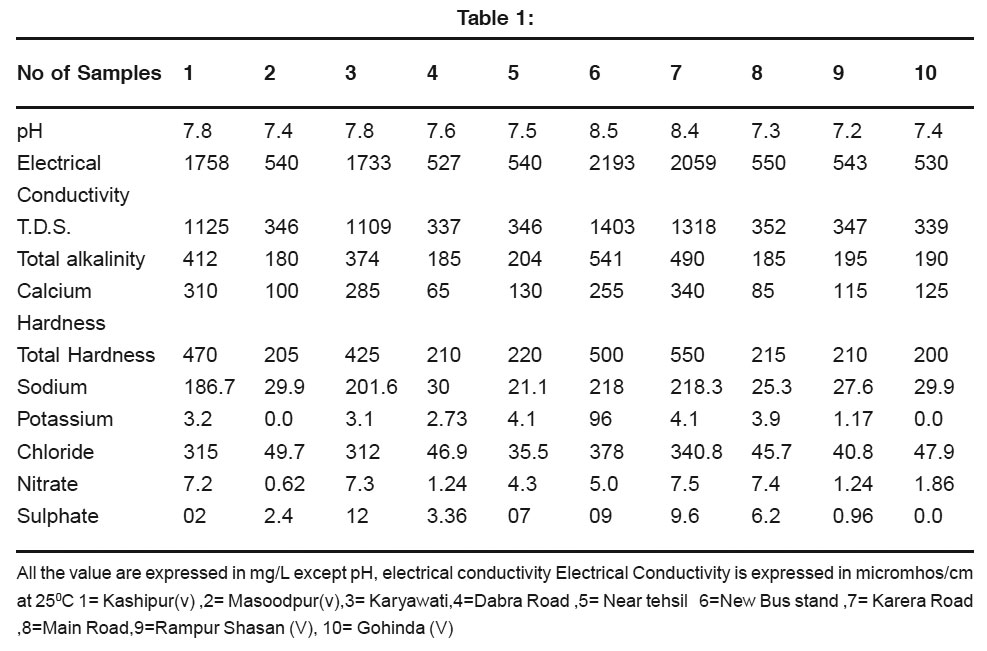 |
Table 1: Click here to view table |
Results and Discussion
pH range of 6.5to 8.5 is normally accepted as per guide line suggested by WHO. The pH value of water sample in the study area ranged from 7.2 to 8.5 .This shows that the pH of water sample was observed to be slightly alkaline .It is known that pH of water does not cause any severe health hazard. The desirable limit of total alkalinity is 200mg/L. The value alkalinity of ground water samples were varied from 180 mg/L to 541mg/L.The desirable limit of total hardness for drinking water according to Indian Standards Institute is 300mg/L.In surveyed area its values in Ground water varied from 200mg/ L to 550 mg/L.
Calcium hardness values in ground water samples varied from 65 mg/L to 340mg/L Sodium and potassium are termed, as alkali metals sodium is abundant in water, because of its compound are readily soluble. In ground water it is generally found to be>5mg per liter. Ground water pollution by sodium salt is an unavoidable phenomenon caused form the return flow of irrigation and disposal of industrial and urban wastes. In large concentration it may affect a person with cardiac diffencies.3 Sodium values in surveyed area varied from 21.1 to 218.3 mg/L.Potassium is an essential nutrient for plants Potassium values in ground water samples varied from 0.0to 96 mg/L Chloride in the form of chloride8 ion is one of the major inorganic anions in water and wastewater. The salty tasted produced by chloride concentrations is variable and dependent on the chemical composition of water. Water containing chloride 250mg /L may have a detectable salty taste. The value of chloride in ground water samples were varied from 35.5 mg/L to 378 mg/L. The chloride is troublesome in irrigation water and harmful for aquatic life. The concentration of Nitrate for supplied water over 100mg/L causes methemoglobinemia particular in infant up to six months of age, Whose main liquid intake is powdered milk formula made up with tap water containing high concentration of nitrates. Nitrate forms nitrosamines in stomach, which causes gastric cancer.4 In all the sample-tested nitrate was within the limit for general use. Its values in ground water samples varied from 0.62 mg/L to 7.5 mg/L Presence of sulphate has less effect on the taste of water compared to the presence of chloride. High value of sulphate above 500mg/L produces bitter taste to water and exertsadverse effect on human.5 In all the samples tested; sulphate was within the limit for general Use. Its values in ground water samples varied from 0.0 mg/L to 12 mg/L E.C. values are responsible to make the criteria of ground water.The Electrical conductivity values of water sample in the study area ranged from 527 to 2193 micromhos/cm at 25°C.
Total solid are considered to be the sum of dissolved and suspended solids. In water sources ,the dissolved solids which usually predominate ,consist mainly of inorganic salts ,small amount of organic matter and dissolved gases the suspended solids contain much of the organic matter any increase there of rends to increase the degrees of pollution of water ,if used for public health purpose. An upper limit 500 ppm has been set in order to control undesirable taste and diarrhea. The permissible limit of TDS suitable for drinking is 500 mg/L (W.H.O.) the total dissolved solids values of water sample in the study area ranged from 337 mg/L to 1403 mg/L.
Acknowledgements
The authors are highly thankful to Dr. Prabha Chauhan and Mr. L.N. Agarwal executive engineer (Div. Ground Water Gwalior), for helpful suggestion
References
1. World Health Organization (WHO), International standard for Drinking water Geneva (1984).
2. Verma S.R., Sharma P., Tyagi A., Rani S., Gupta A.k. and Dalela R.C., Limnologica (Berlin), (1984) 15: 69.
3. Lehr J.H., Gass T.E., petty johan W.A. and De Maree,Domestic water treatment, McGrawhill book Co.(1980).
4. Sengupta R. and Kurishy T.W., Water pollution Gyanodaya prakashan, Nainital 165 (1989).
5. NRC(National Research council), Drinking water and public health ,volno1, safe drinking water committee, National Academy press, Washington D.C. (1997).
6. Bethouex and Rudd, Strategy of pollution control, John Wiley sons, New York ,Santa Barbara, Landon, Sydney,Toronto, (1976) 34-35.
7. Ramacharamoorthy T., Nature Environment and Pollution Technology, (2006) 5(01): 41-46.
8. Chauhan, K.P.S., Kadam ,D.S. and Singh, Naveen Kumar, Ultra Chemistry, (2005) 1(2): 119-121.
9. Singh, Naveen Kumar and Kadam, D.S., Ultra Chemistry, (2006) 2(2): 229-231.
10. Singh ,Naveen Kumar ,Chauhan K.P.S. and Kadam D.S., Ultra Chemistry (2006) 2(2): 227- 228.
11. Singh ,Naveen Kumar and Kadam, D.S,Int. J Chem.Sci (2007)5(2): 592-596.
12. Singh ,Naveen Kumar and Chauhan K.P.S., Current World enviroment: (2007) 2(2): 253-254.






