The Potential Use of Plant Growth Promoting Rhizobacteria (PGPR) for Medicinal Plant Cultivation in Meghalaya: A Review
1
Department of Botany,
University of Science and Technology,
Meghalaya
India
Corresponding author Email: dhriti.delhi@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.18.3.04
Copy the following to cite this article:
Chanda D, Sharma G. D, Ibnat M, Jamir T. M. The Potential Use of Plant Growth Promoting Rhizobacteria (PGPR) for Medicinal Plant Cultivation in Meghalaya: A Review Curr World Environ 2023;18(3). DOI:http://dx.doi.org/10.12944/CWE.18.3.04
Copy the following to cite this URL:
Chanda D, Sharma G. D, Ibnat M, Jamir T. M. The Potential Use of Plant Growth Promoting Rhizobacteria (PGPR) for Medicinal Plant Cultivation in Meghalaya: A Review Curr World Environ 2023;18(3).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-04-24 |
|---|---|
| Accepted: | 2023-11-13 |
| Reviewed by: | 
 Rishikesh Singh
Rishikesh Singh
|
| Second Review by: |

 Bhavana Tomar
Bhavana Tomar
|
| Final Approval by: | Dr. Rui Alexandre Castanho |
Introduction
From the ancient times, people have been depended on natural remedies especially derived from plants for producing drugs to cure various diseases which were based on some traditional knowledge and practice 1. Rhizospheric bacteria helps to promote the growth and resistance to host from various pathogens and abiotic stress conditions. They have the ability to synthesize the secondary metabolites which are found to show positive biological effects2. At present, a significant number of phytotherapeutic compounds are isolated from the beneficial microorganisms which are associated with the host 3.
These remedies, often referred to as medicinal plants or herbs, have been used for their healing properties for thousands of years. India, is known for its diverse culture, geography and climate where a very good number of medicinal plants were utilized by practitioners as herbal medicine from one generation to another generations4-6. In recent times, a great number of growth promoting rhizobacteria (PGPR) are being used for the formulations for commercial purposes which is considered as an affordable and safe microbiological method to reduce the reliance on chemical inputs in the cultivation of medicinal plants 7
According to the Global Strategy for Plant Conservation 2011-2020 of the Convention on Biological Diversity, conservation and assessment of the flora of entire Earth is estimated. International Union for Conservation of Nature (IUCN) listed the threatened species of Meghalaya (Fig.1)9.
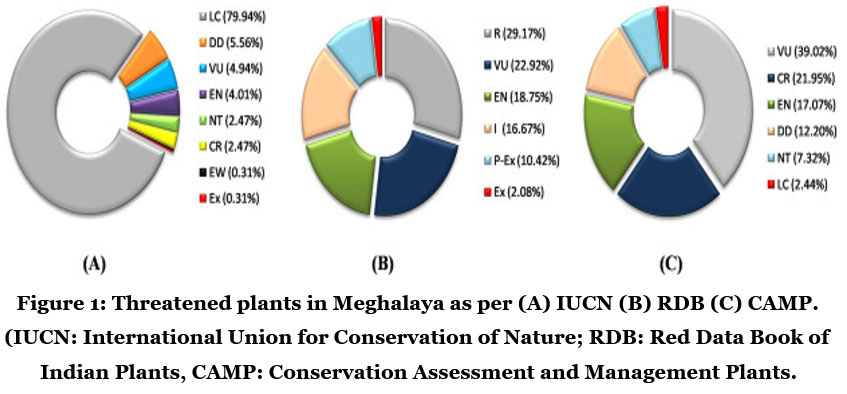 | Figure 1: Threatened plants in Meghalaya as per (A) IUCN (B) RDB (C) CAMP. (IUCN: International Union for Conservation of Nature; RDB: Red Data Book of Indian Plants, CAMP: Conservation Assessment and Management Plants.
|
Microorganisms and medicinal plants
The phytochemicals and antimicrobial properties of extracts obtained from different medicinal in combination with beneficial PGPR strains plants showed significant result to check various pathogenic attack of the host plants. The potential synergistic effects have been observed when most potent plant extracts are combined with suitable antibiotic treatment 10. Numerous studies have investigated the impact of bio-stimulants, such as microorganism, rhizobacteria for in-vitro production of medicinal plants 11-13.
Numerous studies indicated that humic substances have a great role in improving the absorption of soil elements and have indirect effects on the chemical and dynamic processes of microorganisms in the rhizosphere, which ultimately alters the interaction between the soil, plant and microbiota 12. The combination of macro and microelements is closely related with the quality of humic substances and thereby facilitation the absorption of nutrients into the host plant 13, 14. Humic substances can be applied through various methods, including soil application, spraying and irrigation 15-17.
The application of humic substances have been found to increase the biosynthesis and metabolism of medicinal plants. As a result, there is an increase in the production of metabolites with biological and medicinal properties and humic substances have been found to enhance the production of metabolites with pharmacological properties 18, 19.
Studies have also demonstrated that using bactericides and fungicides can effectively lower the incidence of P. notoginseng root rot disease. However, the use of these agents can potentially lead to residual bactericides and fungicides which could pose a threat to the safety of herbal medicine 20,21. The introduction and colonization of biocontrol microorganisms to combat root-rot disease has been widely reported as an alternative of environment friendly cultural practices .This method involves inoculating the plant with microorganisms that can suppress the growth and activity of root-rot pathogens, effectively reducing the incidence of P. notoginseng root-rot disease 22-24.The diverse metabolic activities of microorganisms make them significant contributors to the biogeochemical cycles. These microorganisms have the ability to transform and cycle arsenic through various processes, highlighting their importance in the natural cycling of this element 25.
Beneficial role of bacteria in medicinal plants
Disease resistance
In crop breeding, CRISPR/Cas9 is used to accelerate the growth of medicinal plant varieties by increasing the length and Guanine-Cytosine content of sgRNA. The CRISPER/Cas9 system mainly focussed on plant genome modifications by introducing specific mutations in coding regions 26.
There are two processes i.e., perception of the pathogen attack, followed by responses to limited disease in gene-to-gene plant interaction 27.CRISPR-Cas is used as molecular scissors in the field of antiviral defence mechanism in the host plants which breaks the substrate of DNA or RNA molecules at targeted specific sequences. (Fig. 2) 28.
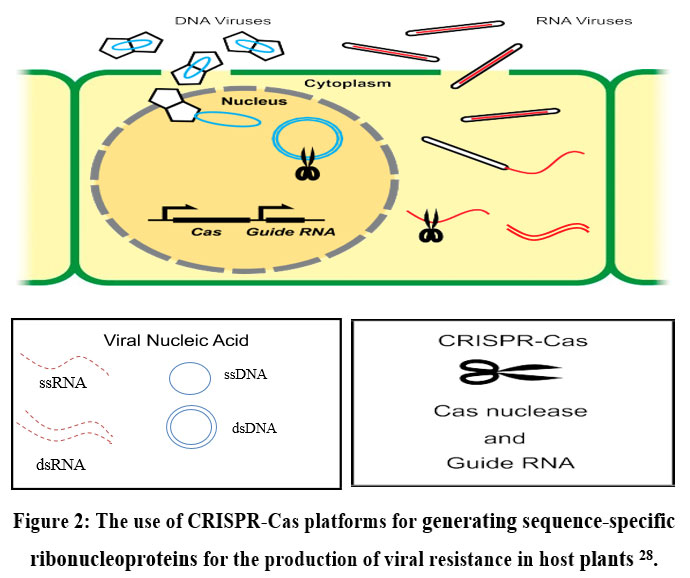 | Figure 2: The use of CRISPR-Cas platforms for generating sequence-specific ribonucleoproteins for the production of viral resistance in host plants 28.
|
The endogenous phytomelatonin accumulation was promoted by application of exogenous melatonin 29, 30. Medicinal plants are very important resources for the synthesis of bioactive molecules. The bacterial endophytes also produce antimicrobial against various plant pathogens for the induction of disease resistance in host plants (Fig.2) 31.
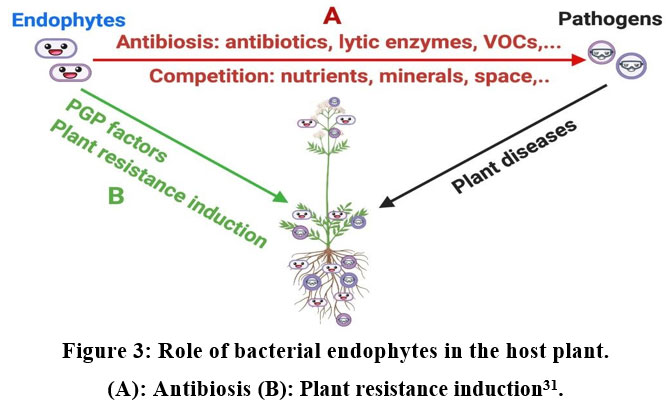 | Figure 3. Role of bacterial endophytes in the host plant. (A): Antibiosis (B): Plant resistance induction31.
|
Stress Resistance
PGPR has been reported as a viable remedy for reducing abiotic stresses and heavy metal contamination in plants 32. It is being reported that application of PGPR enhances the IAA content by increasing the abiotic stress tolerance in wheat 33.
Study demonstrated that diverse group of endophytes which are growing in wild populations are able to reduce the salinity stress by inhibiting the growth of Fusarium oxysporum pathogenesis which is regarded as an excellent tool for the production of biofertilizers and biocontrol agents 34. The antagonistic rhizobacteria is found to be useful for biocontrol strategies in improving cropping systems 35-37. A good number of PGPR were found to be associated with the genera like Azotobacter, Pseudomonas, Bacillus, Mycobacterium, Methylobacterium, Brevibacterium, and Serratia which enhances the overall plant growth by producing secondary metabolites and protects the host plant against the attack of various plant pathogens 38.
Growth yield
The abiotic stress factors can lead to significant growth and yield reductions in the medicinal plants.Certain types of rhizobacteria enhance the growth and development of host plants directly by the supply of various phytohormones through the siderophores and uptake of soluble phosphates 39. Plants engage in communication and interaction with a diverse range of microorganism, releasing a range of substances such as organic acids, water soluble sugars, phenolic compounds and hormones and other metabolites into the rhizospheric region of the soil for the nourishment of beneficial microorganisms. The rhizosphere is characterized by a high concentration of nutrients, which attracts a rich diversity of microorganisms, leading to increased interactions between these microbes 40,41.
Microorganisms present in the rhizosphere are known to impact plant growth through various mechanisms and recycling of soil macro and micronutrients. The various species of Acidobacterium, Bacillus. and Cellulomonas sp. are particularly efficient in the recycling of plant polysaccharides by breaking down these complex compounds into similar forms and thereby helps in the absorption by the roots of the host plant.42-44.
Secondary metabolites possessing antimicrobial properties can be produced by microorganisms. One such example is the antimicrobial activities showed by Burkholderia sp. through the generation of pyrrolnitrin against the various plant pathogens like Fusarium oxysporum, Phytophthora capsici 45.In the context of medicinal plants, PGPR are active biological agents in the development of their hosts pharmaceutical properties or even be the primary source of such properties through a range of mechanisms such as nitrogen fixation, phosphate solubilisation 46-48.
Commercialization of Medicinal Plants
The separation, quantification and identification of plant extracts pose a complex challenge for the generation of medicinal plant products for commercial practices. To overcome these challenges, various techniques as well as detectors have been formulated to enhance the efficiency and accuracy of extract separation. It aims to improve selectively, sensitively and speed during the separation process, facilitating the commercialization of medicinal plant products 49.As such, medicinal plant is a critical resource in promoting the well-being of both human and environmental health 50, 51.
The use of herbal medicines with historical roots is deeply embedded in the indigenous knowledge systems. These systems are integral in determining the use of medicinal plants and are an essential part of the local community’s way of life52.The majority of individuals in the developed countries believe on traditional medicine as their primary source in healthcare practices. This form of medicine has been passed down through generations and is deeply ingrained in the culture and beliefs of these societies 53,54. Pharmaceutical products in industrialized nations also have an indirect reliance on medicinal plants. Many modern medicines are derived from natural plant compounds, with research continually exploring new plant-based treatments. As such, there is a strong connection between traditional medicine and modern pharmaceuticals 55.With over 38,660 species of medicinal plants, Asia is a major hub of bioresource centres worldwide. The vast variety of medicinal plants in Asia makes it an essential resource for the healthcare industry 56, 57.
The commercialization of medicinal plants is crucial for generating income and sustaining livelihoods, as well as being linked to the socio-cultural fabric of communities 58. The utilization of medicinal plants holds significant importance in urban areas, both from an economic and social perspective. It is an essential aspect of healthcare and can provide economic opportunities for those involved in the industry59.It has been suggested that individuals with greater economic means would predominantly buy medicinal plants, while those with fewer resources would gather plants from the wild or employ alternative cultivation methods 60,61.
Limitations and Future prospect
For sustainable agricultural practices, PGPR is considered as the important candidate. The PGPR are regarded as the environmentally friendly for the better yield in the crop. These mechanisms includes hormonal regulations, nutritional balance, inducing disease resistance etc. The study the mechanism of the PGPR for plant growth, extensive future research work is very much necessary to understand the biochemical potential of useful PGPR strains in the field of medicinal plant production. Private-public partnership for increased knowledge and improved future training is also very much necessary in the region of Meghalaya for the sustainable development and maintenance of the rare medicinal by the use of PGPR fertilizer. This review also emphasizes on the future prospects of the beneficial use of PGPR biofertilizer for the cultivation of rare and endangered medicinal plants of Meghalaya.
Conclusion
The utilization of different PGPR formulations for commercial purposes has gained significant popularity in recent times. It is considered as a cost-effective and secure microbiological technique to decrease the dependency on chemical inputs during the growth of medicinal plants. The rhizosphere is a well- known environment that harbors diverse microorganism for the overall growth in the host plants. These microorganisms are found to influence plant growth through multiple mechanisms, including facilitating the recycling of soil nutrients and enhancing the uptake of the essential elements by the plants. It is considered that the use of beneficial strains PGPR will be very much beneficial for the maintenance of sustainable ecosystem. Thus, future research work is very much needed to use the beneficial strains of PGPR and thereby to reduce the application of pesticide especially in the cultivation of medicinal plants in Meghalaya.
Acknowledgement
The authors are grateful to the Department of Botany, University of Science and Technology Meghalaya for providing the laboratory facilities.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
Authors have not received any grant or funding during course of this research.
References
- Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn. Rev.2012; 6: 1-5.
CrossRef - Wu W., Chen W., Liu S., Wu J., Zhu Y., Qin L., Zhu B. Beneficial relationships between endophytic bacteria and medicinal plants. Front. Plant Sci. 2021; 12: 646146.
CrossRef - Köberl M., Schmidt R., Ramadan E M., Bauer R., Berg G. The microbiome of medicinal plants: diversity and importance for plant growth, quality and health. Front. Microbiol. 2013; 4: 400.
CrossRef - Chowti PS., Rudrapur S., Naik BK. Production scenario of medicinal and aromatic crops in India. 2018. J. Pharmacogn. Phytochem; 274-277.
- Sharifi-Rad J., Salehi B., Stojanovi?-Radi? ZZ., Fokou PVT., Sharifi-Rad M., Mahady, GB., Sharifi-Rad M., Masjedi MR., Lawal TO. Ayatollahi S.A. Medicinal plants used in the treatment of tuberculosis-ethnobotanical and ethnopharmacological approaches. Biotechnol. Adv. 2020; 44: 107629.
CrossRef - Mintah SO., Asafo-Agyei T., Archer MA., Junior PA., Boamah D., Kumadoh D., Appiah, A., Ocloo A., Boakye YD., Agyare, C. 2019 Medicinal Plants for Treatment of Prevalent Diseases. In Pharmacognosy-Medicinal Plants; Intech Open: Rijeka, Croatia.
- Karagöz FP., Dursun A. Effects of different PGPR formulations, chemical fertilizers and their combinations on some plant growth characteristics of Poinsettia. Yüzüncü Y?l Üniversitesi Tar?m Bilimleri Derg.2019; 29: 9-15.
CrossRef - Yang L., Yang C., Li C., Zhao Q., Liu L., Fang X., Chen XY. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci. Bull. 2016; 61: 3-17.
CrossRef - Lasushe KA., Mir H., Singh; PP Chaudhary; KL., Choudhury H., Deori C., Roy DK. A comprehensive checklist of threatened plants of Meghalaya, Northeast India. J. Asia-Pac. Biodivers. 2022; 15(3):435-441,
CrossRef - Gupta D., Dubey J., Kumar M. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected common human pathogenic microorganisms. Asian Pac J Trop Dis.2016. 6(1): 15-20.
CrossRef - Halpern M., Bar-Tal A., Ofek M., Minz D., Muller T. Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015; 130: 141-174.
CrossRef - Canellas L P., Olivares FL., Aguiar NO., Jones DL., Nebbioso A., Mazzei P. Piccolo A. Humic and fulvic acids as biostimulants in horticulture. Scientia Horticulturae.2015; 196: 15-27.
CrossRef - Olivares FL. Plant growth promoting bacterium and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017; 4: 1.
CrossRef - Silva Lima L., Olivares FL., Rodrigues R., Vega MRG., Aguiar NO. Canellas LP. Root exudate profiling of maize seedlings inoculated with Herbaspirillum seropedicae and humic acids. Chem. Biol. Technol. Agric.2014; 1:23.
CrossRef - Ko?odziej B., Sugier D., Bieli?ska E. The effect of leonardite application and various plantation modalities on yielding and quality of roseroot (Rhodiola rosea L.) and soil enzymatic activity. J Geochem.Explor.2013;129: 64-69.
CrossRef - Hendawy SF., Hussein MS., El-Gohary AE., Ibrahim ME. Effect of foliar organic fertilization on the growth, yield and oil content of Mentha Piperita Var. Citrata. Asian J. Agric. Res. 2015; 9: 237-248.
CrossRef - Ghasemi K. Antioxidant properties of garlic as affected by selenium and humic acid treatments. N. Z. J. Crop Hortic. Sci. 2015; 43:173-181.
CrossRef - Andrade FMC. Effect of homeopathy on growth and yield of coumarin in “chambá” (Justicia pectoralis Jacq.). Revista Brasileira de Plantas Medicinais. 2001; 4:19-28.
- Khazaie HR., Rezaie EE. Bannayan, M. Application times and concentration of humic acid impact on aboveground biomass and oil production of hyssop (Hyssopus officinalis). J. Med. Plant Res. 2011; 5: 5148-5154.
- Fu Y., Dou X., Lu Q., Qin J., Luo J. Yang, M. Comprehensive assessment for the residual characteristics and degradation kinetics of pesticides in Panax notoginseng and planting soil. Sci Total Environ.2020; 714:136718.
CrossRef - Zhao L., Li Y., Ren W., Huang Y., Wang X., Fu Z., Ma W., Teng Y. Luo Y. Pesticide residues in soils planted with Panax notoginseng in south China, and their relationships in Panax notoginseng and soil. Ecotoxicol. Environ. Saf .2020; 201:110783.
CrossRef - Chen JL., Sun SZ., Miao CP., Wu K., Chen YW., Xu LH., Guan HL. Zhao LX. 2016b.Endophytic Trichoderma gamsii YIM PH30019: a promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res.2016b; 40:315-324.
CrossRef - Fan ZY., Miao CP., Qiao XG., Zheng YK., Chen HH., Chen YW., Xu LH., Zhao LX. Guan HL. Diversity, distribution, and antagonistic activities of rhizobacteria of Panax notoginseng. J. Ginseng Res.2016; 40: 97-104.
CrossRef - Miao CP., Mi QL., Qiao XG., Zheng YK., Chen YW., Xu LH., Guan HL. Zhao LX. 2016.Rhizospheric fungi of Panax notoginseng: diversity and antagonism to host phytopathogens. J. Ginseng Res.2016; 40:127- 134.
CrossRef - Mallick I., Islam E., Mukherjee SK. Fundamentals and application potential of arsenic-resistant bacteria for bioremediation in rhizosphere: a review. Soil Sediment Contam. 2015; 24:704-718.
CrossRef - Wan HF., Han WJ., Zhou L., Wang S. Sui C. New Advances of CRISPR/Cas9 Technique and its Application in Disease Treatment and Medicinal Plants Research. Curr. Pharm. Biotechnol. 2022; 23(14): 1678-1690.
CrossRef - Ellis J., Dodds P., Pryor T. Structure, function and evolution of plant disease resistance genes. Curr. Opin. Plant Biol.2000; 3(4): 278-284.
CrossRef - Dong OX., Ronald PC. Genetic engineering for disease resistance in plants: recent progress and future perspectives. Plant Physiol.2019; 180(1):26-38.
CrossRef - Yang Q., Li J., Ma W., Zhang S., Hou S., Wang Z., Cui X. Melatonin increases leaf disease resistance and saponin biosynthesis in Panax notogiseng. J. Plant Physiol. 2021; 263: 153466.
CrossRef - Yang X., Xiao Y., Wang X., Pei Y. Expression of a novel small antimicrobial protein from the seeds of motherwort (Leonurus japonicus) confers disease resistance in tobacco. Appl. Environ. Microbiol. 2007; 73(3): 939-946.
CrossRef - Castronovo LM., Vassallo A., Mengoni A., Miceli E., Bogani P., Firenzuoli F. Maggini V. Medicinal plants and their bacterial microbiota: A review on antimicrobial compounds production for plant and human health. Pathogens.2021; 10(2): 106.
CrossRef - Dutta, S., Khurana SP. Plant growth-promoting rhizobacteria for alleviating abiotic stresses in medicinal plants. Plant-growth-promoting rhizobacteria (PGPR) and medicinal plants, 2015;167-200.
CrossRef - Barnawal D., Bharti N., Pandey SS., Pandey A., Chanotiya CS., Kalra A. Plant growth?promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiologia plantarum, 2017; 161(4): 502-514.
CrossRef - Abdelshafy Mohamad OA., Ma JB., Liu YH., Zhang D., Hua S., Bhute S., Li L. Beneficial endophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Front. Plant Sci.2020; 11: 47.
CrossRef - Beneduzi A., Ambrosini A., Passaglia LM. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012; 35: 1044-1051.
CrossRef - Ogbe AA., Finnie JF., Van Staden J. The role of endophytes in secondary metabolites accumulation in medicinal plants under abiotic stress. S. Afr. J. Bot.2020; 134: 126-134.
CrossRef - Kumar R., Swapnil P., Meena M., Selpair S., Yadav BG. Plant Growth-Promoting Rhizobacteria (PGPR): Approaches to alleviate abiotic stresses for enhancement of growth and development of medicinal plants. Sustainability. 2022; 14(23): 15514.
CrossRef - Vaghela N., Gohel S. Medicinal plant?associated rhizobacteria enhance the production of pharmaceutically important bioactive compounds under abiotic stress conditions. J. Basic Microbiol. 2023; 63(3-4): 308-325.
CrossRef - Arun MN., Hebbar SS., Bhanuprakash T., Nair AK., Padmavathi G., Pandey P., Singh A. 2022.Seed priming: The way forward to mitigate abiotic stress in crops. In Plant Stress Physiology-Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK.
- Sasse J. Martinoia, E. Northen T. Feed your friends: do plant exudates shape the root microbiome ? Trends Plant Sci. 2018; 23:25-41.
CrossRef - Yu P., Hochholdinger F. The role of host genetic signatures on root–microbe interactions in the rhizosphere and endosphere. Front. Plant Sci. 2018; 9:1896.
CrossRef - López-Mondéjar R., Zühlke D., Becher D., Riedel K., Baldrian P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016; 6:25279.
CrossRef - Poulsen HV., Willink FW., Ingvorsen K. Aerobic and anaerobic cellulase production by Cellulomonas uda. Arch. Microbiol. 2016; 198: 725-735.
CrossRef - Belova SE. Ravin NV. Pankratov TA. Rakitin AL. Ivanova AA. Beletsky AV. Hydrolytic capabilities as a key to environmental success: chitinolytic and cellulolytic Acidobacteria from acidic sub-arctic soils and boreal peatlands. Front. Microbiol. 2018; 9:2775.
CrossRef - Jung BK., Hong SJ., Park GS., Kim MC., Shin JH. Isolation of Burkholderia cepacia JBK9 with plant growth promoting activity while producing pyrrolnitrin antagonistic to plant fungal diseases. Appl. Biol. Chem. 2018; 61: 173-180.
CrossRef - Adeleke BS., Babalola OO. Pharmacological potential of fungal endophytes associated with medicinal plants: A Review. J. Fungi.2021; 7: 147.
CrossRef - Maggini V., De Leo M., Mengoni A., Gallo E., Miceli E., Reidel, R. Plant-endophytes interaction influences the secondary metabolism in Echinacea purpurea (L.) Moench: an in vitro model. Sci. Rep. 2017;7: 16924.
CrossRef - Bhattacharya PN. Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol Biotechnol.2012; 28:1327-1350.
CrossRef - Masondo NA., Makunga NP. Advancement of analytical techniques in some South African commercialized medicinal plants: Current and future perspectives. S. Afr. J.Bot.2019; 126 : 40-57.
CrossRef - Larsen HO., Smith PD., Olsen CS. Nepal’s conservation policy options for commercial medicinal plant harvesting: Stakeholder views. Oryx.2005; 39: 435.
CrossRef - Yang L. Ahmed S., Stepp JR., Mi K., Zhao Y., Ma J., Liang C., Pei S., Huai H. Xu G. Comparative homegarden medical ethnobotany of Naxi healers and farmers in Northwestern Yunnan, China. J. Ethnobiol. Ethnomed.2014; 10: 6.
CrossRef - Torri MC., Herrmann, TM.2011. Bridges between tradition and innovation in ethnomedicine. Fostering local development through community-based enterprises in India; Springer: Heidelberg, Germany. ISBN 978-94-007-1112-9.
CrossRef - Jeelani S.M., Rather G.A. Sharma, A. Lattoo S.K. In perspective: Potential medicinal plant resources of Kashmir Himalayas, their domestication and cultivation for commercial exploitation. J. Appl. Res. Med. Aromat. Plants.2018; 8: 10-25.
CrossRef - Karunamoorthi K., Jegajeevanram K., Vijayalakshmi J. Mengistie E.. Traditional medicinal plants: A source of phytotherapeutic modality in resource-constrained health care settings. J. Evid. Based Complement. Altern. Med.2013; 18: 67-74.
CrossRef - Munasinghe M.. Making Development More Sustainable: Sustainomics Framework and Practical Applications; MIND Press: Colombo, Sri Lanka, 2010 ISBN. 978-955-0317-00-4.
- Zuhud EA. The Indonesian tropical forest as buffer of natural medicine product for national healthy. J. Bahan Alam Indones.2009; 6: 227-232.
- Phumthum M., Srith K., Inta A., Junsongduang A. Ethnomedicinal plant diversity in Thailand. J. Ethnopharmacol. 2017;214: 90-98.
CrossRef - Astutik S., Pretzsch J. Ndzifon- Kimengsi J. Asian medicinal plants’ production and utilization potentials. Sustainability .2019; 11: 5483.
CrossRef - Gaoue OG., Coe MA., Bond M., Hart G., Seyler, BC., McMillen H. Theories and major hypotheses in ethnobotany. Econ. Bot.2017; 71: 269-287.
CrossRef - Ramet A., Benyei P., Parada, M., Aceituno-Mata L., García-del-Amo D., Reyes-García V. Grandparents’ proximity and children’s traditional medicinal plant knowledge: Insights from two schools in intermediate-rural Spain. J. Ethnobiol.2018; 38:187-204.
CrossRef - Rizvi A., Ahmed B., Khan MS., El-Beltagi HS., Umar S., Lee J. Bioprospecting plant growth promoting rhizobacteria for enhancing the biological properties and phytochemical composition of medicinally important crops. Molecules. 2022;19: 27(4):1407.
CrossRef







