A study of effectiveness of an inorganic heterocycle (S6N4)2+ Cl2- in reclamation of acidic soil of Andaman and Nicobar islands
H.K. Sharma1 *
1
Department of Chemistry,
J.N.R. Mahjavidhyalaya,
Port Blair,
744 104
India
DOI: http://dx.doi.org/10.12944/CWE.4.1.16
Cyclohexa thiazenium chloride (S6N4)2+Cl2- has been tested against varying pH 3 – 4.5 of acidic soil of Andaman and Nicobar islands. It has been observed that there were remarkable increase in pH 7.4 in normal time.
Copy the following to cite this article:
Sharma H.K. A study of effectiveness of an inorganic heterocycle (S6N4)2+ Cl2 - in reclamation of acidic soil of Andaman and Nicobar islands. Curr World Environ 2009;4(1):113-115 DOI:http://dx.doi.org/10.12944/CWE.4.1.16
Copy the following to cite this URL:
Sharma H.K. A study of effectiveness of an inorganic heterocycle (S6N4)2+ Cl2 - in reclamation of acidic soil of Andaman and Nicobar islands. Curr World Environ 2009;4(1):113-115. Available from: http://www.cwejournal.org/?p=905
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-12-10 |
|---|---|
| Accepted: | 2009-01-21 |
Introduction
The soil of Andaman and Nicobar islands is affected by the halogen salts of alkali metals. The major content of the salt adsorbed on the land surface is leached out by heavy rainwater. Even though few contents of the salt remain adsorbed on the soil resulting the soil to be acidic. Few inorganic hetercyclics like tetrasulphurtetranitride (S4N4), thiotrithiazyl chloride (S4N3)+Cl- have been successfully tested in reclamation of alkaline (Sharma, 1986, 2000) as well as acidic (Sharma, 1994) and to reclame alkaline soil (Sharma et al, 1994) has already been investigated. To explore the further utility of (S6N4)2+Cl2- in agriculture, a study of effectiveness of (S6N4)2+Cl2- on varying pH of acidic soil of Andaman and Nicobar islands is being presented here.
Material and Methods
(S6N4)2+Cl2- was prepared (Goehring, 1957) by refluxing S2Cl2 and NH4Cl in nitromethane. The sequence of reaction in the preparation are:
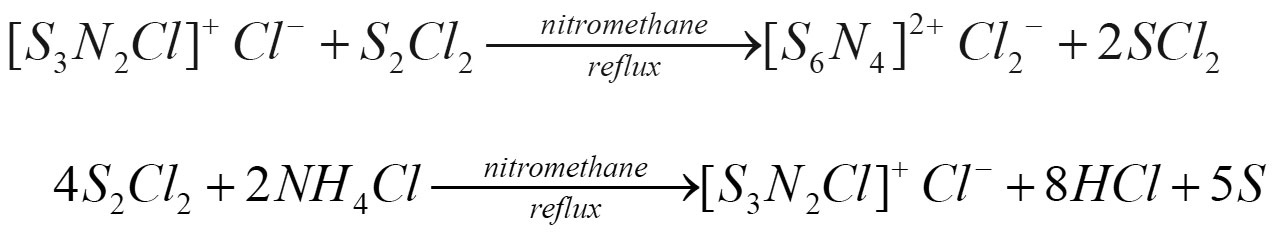
Black green coloured product formed was separated and washed with distilled water, nitromethane and CS2 to remove unreachted NH4Cl, S2Cl2 and S. The formed product being soluble in water, nitromethane supports its ionic nature (Goehring, 1970), was dried in vacuo. To study the effect of (S6N4)2+ Cl2- on varying pH 3 - 4.5 of acidic soil, the soil samples from different places of Andaman and Nicobar islands namely Mayanbunder, Bambooflat and Miletilak were collected upto desired depth 15 cm by means of sampling tools. Each soil sample (20 gm) was taken in 100 ml beaker to which 40 ml distilled water was added. The suspension was stirred at regular intervals for 30 minutes. To determine the pH, the each suspension was stirred well just before the electrodes were immersed and then the pH of each extract was noted before and after each addition of fixed quantity (10 mg) of (S6N4)2+ Cl2- at 10 minutes time interval. In each determination, the electrodes were washed with distilled water and water was removed from the surface with a piece of filter paper. Different ions present in the soil sample were analysed by spot tests (Feizi Fritz, 1957).
 |
Table 1: Change in pH of soils on adding (S6N4)2+ Cl2- Click here to view table |
Results and Discussion
Quantities analysis of soil samples indicates that soil samples contain Cl-, Zn2+, Fe2+ and Na+ ions. The acidic characters of soil may be due to Cl- ions. Zn2+ and Fe2+ show amphoteric character in aqueous extract of soil sample. (S6N4)2+ Cl2- having three planer fused rings possesses cyclic structure (Banister 1974, 1975). The S – S bonds (303 pm) holding the two S- N (161 pm) rings together and forming the middle ring are very long. (S6N4)2+ Cl2- undergoes hydrolysis in acidic soil as:
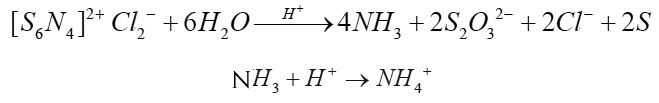
The hydrolysis products were analysed qualitatively and quantitatively by standard methods (Vogel, 1968). The nitrogen is completely converted into NH3 confirming the physical structural deduction that there is no N – N bond in the molecule but there are S- N and S – S bonds. During the present study (S6N4)2+ Cl2- used against acidic soils of different pH 3 – 4.5 show a remarkable increase in pH of acidic soil (Table 1) and the pH has increased upto 7.4 in normal time. There is no further increase in pH of the soil indicating that after retaining pH 7.4, (S6N4)2+ Cl2- creates a buffer state due to formation of NH4Cl and NH4OH during the hydrolysis in acidic soil.
S2O32-, Cl- ions formed during hydrolysis of (S6N4)2+ Cl2- are exchanged by Zn2+, Fe2+ and Na+.
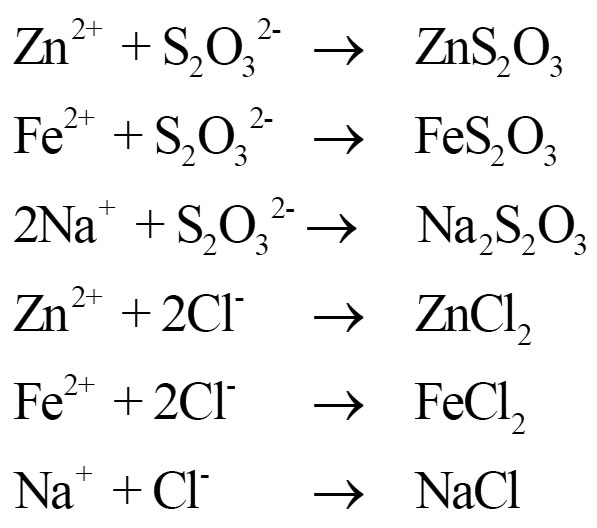
(S6N4)2+ Cl2- is a suitable chemical to reclame acidic soil because.
- It checks the acidity and increases the pH of soil upto 7.4 which is a most suitable state for farming.
- The main ions responsible for acidity are Cl-ions. These ions are taken up by NH4+ ions of compound formed during the hydrolysis of (S6N4)2+ Cl2-.
Since ammonia is evolved during the hydrolysis of (S6N4)2+ Cl2- in acidic medium it may be a good fertilizer to make up N – deficiency in acidic soil as well as in normal soil which remains unaffected.
References
- Banister, A.J., Clarke, H.G., Rayment, I and Shearer, H.M.M., Inorg. NuCl, Chem., Lett., (1974) 10: 647.
- Banister, A.J., M.T.P., International Review of Science, Inorganic Chemistry Series 2, (1975) 3: 41.
- Feizi Fritz, ‘Spot tests in inorganic analysis’ Elsevier Publication, London (1958).
- Goehring, M.B., Inorg. Macromol Rev., (1970) 17.
- Goehring, M.B., Ergebnesse and Probleme der Chemie der Schwefelstickstoff – Verbindungen, Akademie – Verlag, Berlin (1957).
- I. Vogel, ‘A text book of quantitative inorganic analysis’, ELBS Publication, London (1968).
- Sharma, H.K. and Jadon, S.P.S. Bio-science Research Bulletin, (1986) 2(1-2): 9-10.
- Sharma, H.K. Bangladesh, J.Sci. Ind. Res., (2000) 35(1-4): 128-129.
- Sharma, H.K., Indian, J. Applied and Pure Bio., (1998) 13(2): 95-97.
- Sharma, H.K. Ecol. Env. A cons., (1999) 5(4): 399-400.
- Sharma, H.K. Banladesh, J. Sci. Ind. Res.,(1994) 29(4): 157-162.
- Sharma, H.K. and Upadhyay, V.K., Ad. Plant Sci., (1994) 7(1): 68-71.






