Chemical analysis of water from different ponds of Newara village of Bilaspur (C.G.) India
Gayatri Neelam 1 * and N.K. Singh1
DOI: http://dx.doi.org/10.12944/CWE.4.1.32
In present investigation samples of water from different ponds of Newara Village of Bilaspur District of Chhattisgarh have been collected and analysed for the presence of various ions, hardness and alkalinity. We report a considerable increase in their values and thereby provide a base for recommendation for no-use of pond water specially for cooking, drinking and bathing.
Copy the following to cite this article:
Shastri G.N, Singh N.K. Chemical analysis of water from different ponds of Newara village of Bilaspur (C.G.) India. Curr World Environ 2009;4(1):191-193 DOI:http://dx.doi.org/10.12944/CWE.4.1.32
Copy the following to cite this URL:
Shastri G.N, Singh N.K. Chemical analysis of water from different ponds of Newara village of Bilaspur (C.G.) India. Curr World Environ 2009;4(1):191-193. Available from: http://www.cwejournal.org/?p=937
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-02-25 |
|---|---|
| Accepted: | 2009-04-29 |
Introduction
A few decades ago, water was considered as plentiful and in-expensive resource. But rapid industrialization, urbanization and agricultural development have made it as a scarce resource.3 Contamination of surface water together with economic development have contributed for pollution of surface water. With growing load of population, the situation is worst in India. Availability of surface and ground water is sharply decreasing day by day.12 In many Indian cities, alternate day water is being supplied for drinking and house hold uses.
There are thousand of villages in our country where drinking water is not available and people have to march kilometers to collect proper drinking water. The situation has compelled the people to use water of ponds and other water bodies for their requirements.6 The newly formed Chhattisgarh state has a number of such villages.
Bilaspur is a city of C.G. and located at 25°5’N latitude and 82025’ longitude. Population of the city is around 4 lacs. The village Newara is situated approximately 22 km. away from the city on Bilaspur-Kota road, Major water requirements of villagers are fulfilled by ponds. There are altogether five ponds in and around the village. These ponds are natural. The pond water is used for drinking, washing clothes and utensils, bathing by man and his cattle, trapa cultivation, fish production and even for irrigation.
The quality of pond water depends on the presence of ions and other organic/inorganic ingredients dissolved in water. Major ions present in pond water are carbonate, bicarbonate, chloride, nitrate and fluoride. These are present as cations and anions and their balance is essential to maintain the water quality. The natural quality of water is rapidly being degraded mainly by man made factors.5 The present study aims at the chemical analysis of water of ponds in the village Newara of Bilaspur.
Material and Methods
During present study, five different ponds of Newara village were selected. Water samples from the ponds were collected in the month of December, 2007. Sample collection was made in sterilized or acid washed plastic bottles. Chemical analysis of water samples was made as soon as possible because prolonged storage of water may change its chemical characteristics. pH of samples was measured by systronic digital pH meter. Total alkalinity was determined by titrating with N/10 HCl using phenolphthalein and methyl orange as an indicator. Total hardness was assessed by complexometric titration with EDTA using eriochrome black-T as an indicator. Likewise, calcium measurement by EDTA titrimetric method, magnesium by total hardness minus calcium, chloride by Argentometric method, nitrate by UV-visible spectrophotometry and measurement of total dissolved substance (TDS) and fluoride were carried out by standard methods described by APHA (1985) and Trivedi and Goel (1986).8
Results and Discussion
The results of present study have been presented in Table 1. pH of water from pond number-3 indicated a slightly higher value (8.8) as the desirable pH range necessary for drinking water is from 7.0 to 8.5. However, the pH of pond water is well within the maximum permissible limit of pH as recommended by WHO (6.9-9.2).10 Samples of other four ponds exhibited normal pH within the range of limit. Total alkalinity in pond water samples ranged from 150.98 to 2178.04. The much higher alkalinity of water from pond number 2 and 5 (983.64 and 217.04, respectively) can quite be compared with normal alkalinity (200 mg/L) recommended by ISI.6 Alkalinity of water from remaining three ponds were within the permissible limit.
As far total hardness is concerned, water samples from all the five ponds exhibited normal values ranging from 156.67 mgL-1 to180 mgL-1. These values are quite below the desirable limit of 300 mgL-1 which is recommended by ISI. Total hardness of water is due to presence of calcium and magnesium salts or ions. Therefore, these two were measured separately in all the water samples collected from ponds. Values of magnesium varied from 5.54 to 28.79 mgL-1 which were within the permissible limit of WHO11 (153. mgL-1). However, calcium content in ponds water ranged from 104.54 mgL-1 to 157.15mgL-1, which are above the permissible value of 75 mgL-1.
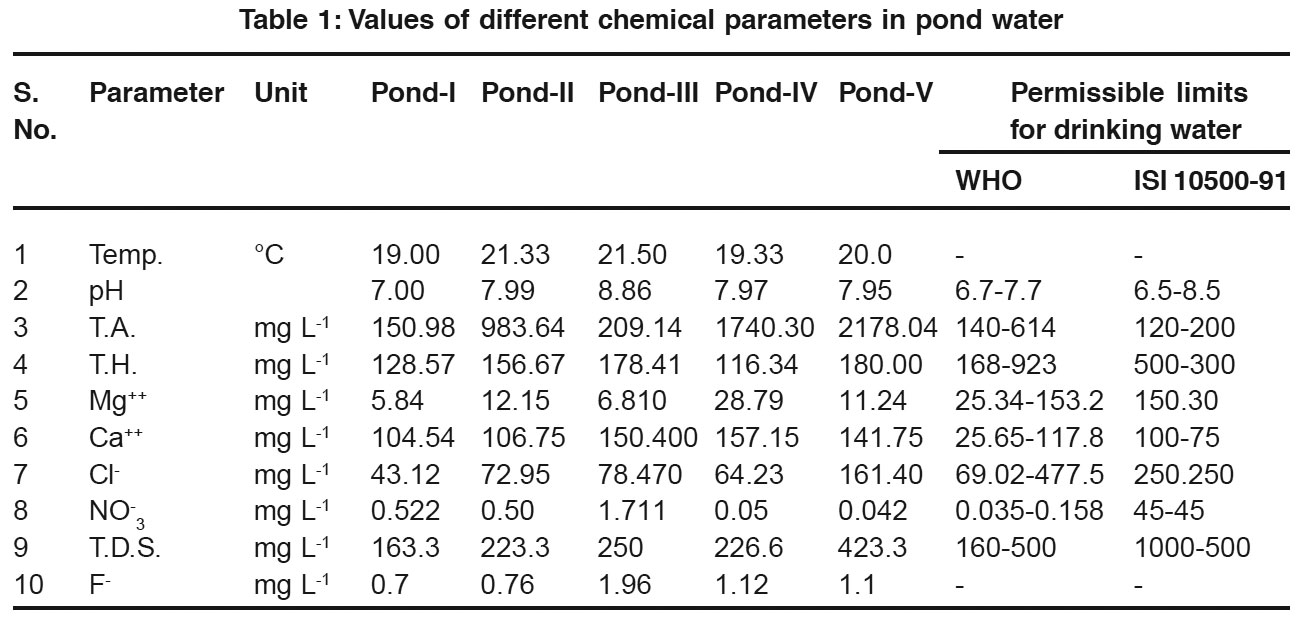 |
Table 1: Values of different chemical parameters in pond water Click here to view table |
Salty taste produced by the presence of chloride concentration is variable and dependent on the chemical composition of water. Water containing 250 mgL-1 chloride may have salty taste. In different samples, chloride values ranged from 43.12 mg L-1 to 161.40 L-1. In recent years, nitrate contamination of drinking water has posed serious problem to human health. It enters the human body through drinking water and cause a number of disorders like methemoglobinemia, gastric cancer, goitre birth malformations, hypertension, blue bady syndrome in children etc.7 Nitrate concentration in ponds water varied from 0.42 mg L-1 to 1.711 mg L-1.
Fluoride present in drinking water is significant from physiological point of view, In human beings and other mammals it causes fluorosis. Specially dental fluorosis is causing serious threat in many parts of India. Many workers have reported that if fluoride concentration in drinking water exceeds 1.5 mgL-1, it causes teeth motling and still higher concentration may lead to skeletal fluorisis.9 During present investigation, fluoride in water samples were found to vary from 0.7 mgL-1 to 1.1 mg L-1. Total dissolved solids (TDS) are an important parameters for use of water for drinking and other purposes. According to WHO report (2004), the limit of TDS in drinking water is 500 mg L-1. In the present study, TDS ranged from 163.3 mg L-1 to 423.3 mgL-1.
Conclusion
The present study has been made to evaluate the chemical properties of water in ponds of Newara village of Bilaspur (C.G.). Selection of the village was mainly due to large scale dependence of villagers on pond water for their house hold daily use including drinking and bathing. The outcome of the present study clearly reveals that at least from chemical contamination point of view the ponds water is contaminated and can never be recommended for drinking and cooking purpose. Even if the supply of pond water to local people is necessary due to socio-political and geological reasons proper treatment of pond water should be done before its supply.
Acknowledgements
The authors are thankful to Dr. (Mrs.) Asha Kaushik, Principal, Govt. E. Raghavendra Rao science P.G. College, Bilaspur (C.G.) for her kind permission to persue doctoral research work (to GNS) in the Department of Botany. Thanks are also to Prof. M.F.K. Khokhar, Retired Principal, Govt. College Masturi, (Bilaspur) for his moral encouragement and intellectual support.
References
- APHA-AWWA- and WPCF, Standard methods for the Examination of water and waste water, 16th edition Amer. Publ. Health Assoc. Inc. Newyork (1985).
- Baruah , B.K., Talukdar,S. and Brotholcur, Area of Kamrup Distt. Assam. Evn. Eco. 8 Con. (1984) 16(2): 254-256.
- Bethouex and Rudd, Strategy of pollution contral, John Wiley Sons, New York, Santa Barbara, London, Sydney, Toronto, (1976) 34-35.
- Chona, M.K., Physico-chemical complexes of a polluted pond at Halomagra (Chandigarh) Himalayan, J. Environ. Zoo, (1991) 5(1): 2-44.
- Grewal, Water quality in punjab, JIWWA (1976) 4275-4279.
- ISI, Indian Standard specification for drinking water, New Delhi, IS. 10500 (1983).
- Olaniya, Pollution Studies of well water in sewage from Jaipur, Indian, J. Environ., (1969) 20(4): 398-412.
- Trivedi, R.K. and Goel, P.K., Chemical and biological methods for water pollution studies, Environ. Pub. Karad, India, (1986) 1-28.
- Verma, S.R., Sharma, P., Tyagi, A., Rani, S., Gupta, A.K. and Dalela, R.C., Limnologica (Berlin), (1984)​​​​​​​ 15: 69.
- World Health Organization (WHO), Environmental Health Criteria, 5, Geneva (1978).
- World Health Organization (WHO), International Standard for Drinking Water, Geneva (1984).
- World Health Organization (WHO), Guidelines for drinking water quality, Geneva, 3rd Edn., 1 (2004).






