Population dynamics and community structure of zooplankton inhabiting in fish pond Jammu, India
M. K. Jyoti1 * , K. K. Sharma1 and Jyoti Sharma1
DOI: http://dx.doi.org/10.12944/CWE.4.1.26
During the present period of investigation extending from March, 2004 to February, 2005, 15 species of zooplankton belonging to five different taxa viz., Protozoa, Rotifera, Copepoda, Ostracoda and Cladocera were collected. Three of these taxa, Protozoa, Copepoda and Ostracoda were represented by single species while Rotifera by eight species and Cladocera by four species besides one larval form, the Nauplius. The number of plankton belonging to these taxa exhibited both quantitative and qualitative changes concuss with seasons and thus resulted in community changes during different seasons. The probable reasons (environment) that stand responsible and result in changes in community structure and individual population of each plankton have been discussed at length
Copy the following to cite this article:
Jyoti M.K, Sharma K.K, Sharma J. Population dynamics and community structure of zooplankton inhabiting in fish pond Jammu, India. Curr World Environ 2009;4(1):165-169 DOI:http://dx.doi.org/10.12944/CWE.4.1.26
Copy the following to cite this URL:
Jyoti M.K, Sharma K.K, Sharma J. Population dynamics and community structure of zooplankton inhabiting in fish pond Jammu, India. Curr World Environ 2009;4(1):165-169. Available from: http://www.cwejournal.org/?p=925
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-05-12 |
|---|---|
| Accepted: | 2008-08-17 |
Introduction
Zooplankton, the microscopic faunal component of lotic and lentic waters serve as an important interlace in the food chain or energy transfer operating in an aquatic ecosystem. Their importance in fishery as live and natural feed for early larval stages is immense. Infact, non availability of right kind of live food at critical period, when fish larvae shift from endogenous to exogenous feeding is responsible for major part of fish biopotential loss (Nikolsky, 1963). This loss in biopotential is primarily due to selective feeding and competition generated between the members of species at their tender age but at the same time indirectly offers equal feeding opportunities to the larvae of different fish living sympatrically and breeding concurrently in a given water. Needless to say species oriented selective feeding at suggested feeding niche amongst species to help evade feeding competition amongst species thereby ensuring healthy new recruitment for continuation of balanced fish population. Therefore study of zooplankton and their analysis regarding seasonal dynamics is not simply a reflection of energy transfer in an aquatic ecosystem but it also certainly unravels the information about the happenings in food chains and may even serve as an important parameters in evaluating fishery potential as well as the kind of fishery that could be practiced in a given water body. This may also help sympatric fish to maintain their population without generating any feeding competition and hence loss of energy. Besides the knowledge about plankton is also important because of their established role as pollution indicators (Kumar and Singh, 1999 and Gaur et al., 1999)
Material and Methods
Fish pond is a concrete man made pond raised in the fish farm of University of Jammu. The pond receives freshwater supplied from tube well source. Its surface area is 157.5 m² and depth of 7 feet with volume 335.48m³. At no given time the pond had water less than 288.225m³. Nutrient source of the pond mainly includes leacheate from the manure. Fishes that has been introduced into the pond includes Tor putitora, Labeo rohita, Cyprinus carpio, Puntius species, Cirrhinus mrigala and Catla catla.
For the study of zooplankton, water sample was collected by filtering 20 liters of water with the help of hand net having a mesh size of 60-70 µm. The samples so collected were fixed by adding 5% formaldehyde solution. Identification of the zooplanktons done following Pennak (1968); Edmondson and Winberg (1971) and Adoni (1985). For quantitative studies zooplanktons were counted by field count method (A.P.H.A.,1976).
Results and Discussion
The total zooplankton inhabiting fish pond was observed to comprise of 15 species belonging to 15 genera, 5 order and 8 families. But at no given time the zooplankton community comprised of more than nine species (Table 1).
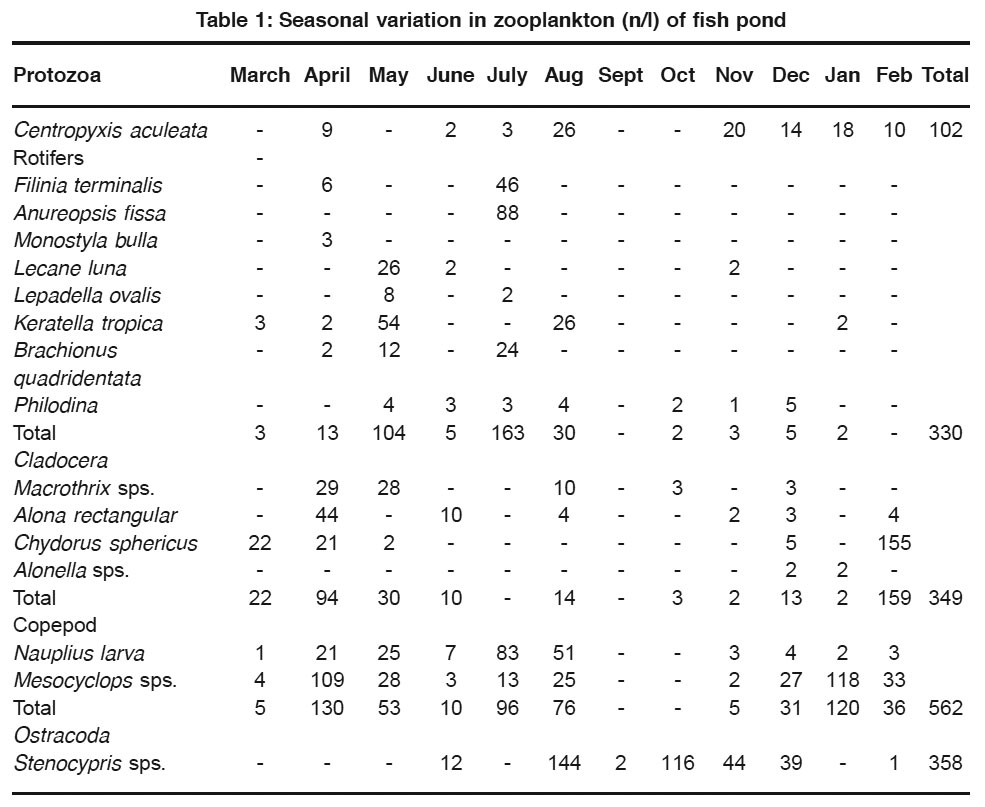 |
Table 1: Seasonal variation in zooplankton (n/l) of fish pond Click here to view table |
The rich zooplankton community with 9 species belong to 9 genera was observed during April while zooplankton represented by the population of solitary species of Ostracoda during September. Quantitative hierarchial representation during different season by different taxa was of the following order:
Spring: Copepoda > Caldocera > Rotifera > Protozoa > Ostracoda.
Summer: Rotifera > Copepoda > Cladocera >
Ostracoda > Protozoa
Monsoon: Rotifera > Copepoda > Ostracoda >
Protozoa > Cladocera
Autumn: Ostracoda > Protozoa > Copepoda =
Cladocera=Rotifera
Winter: Copepoda > Cladocera > Protozoa >
Ostracoda > Rotifera
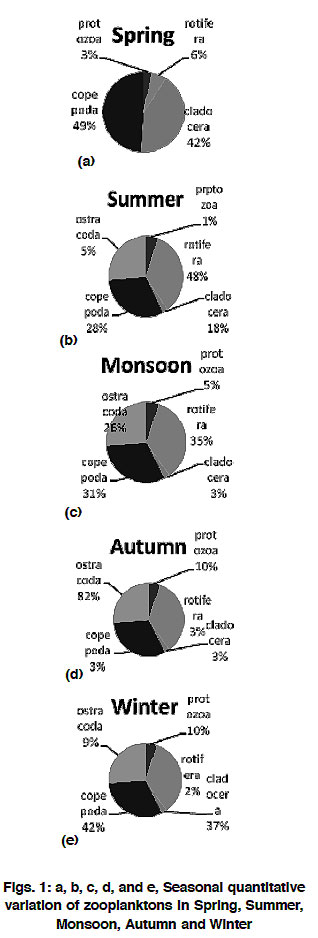 |
Figure 1: a, b, c, d, and e, Seasonal quantitative variation of zooplanktons in Spring, Summer, Monsoon, Autumn and Winter Click here to view figure |
On the contrary the qualitative (number of species) contribution to community during different season was of the order:
Spring: Rotifera > Caldocera > Protozoa =
Copepoda
Summer: Rotifera > Cladocera > Protozoa =
Copepoda = Ostracoda
Monsoon: Rotifera > Cladocera > Protozoa =
Copepoda = Ostracoda
Autumn: Rotifera = Cladocera > Copepoda =
Protozoa = Ostracoda
Winter: Cladocera > Rotifera > Copepoda =
Protozoa = Ostracoda
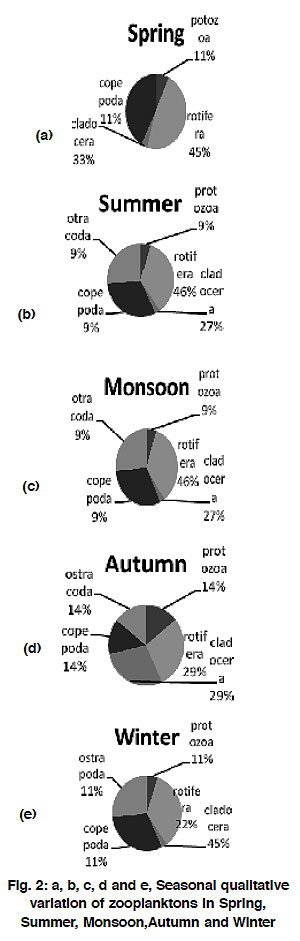 |
Figure 2: a, b, c, d and e, Seasonal qualitative variation of zooplanktons in Spring, Summer, Monsoon,Autumn and Winter Click here to view figure |
Thus, Rotifera as a group was dominating quantitatively as well as qualitatively and was represented by eight species viz., Filinia terminalis, Anureopsis fissa, Monostylla bulla, Lecane luna, Lepadella ovalis, Keratella tropica, Brachionus quadridentata and Philodina species, Cladocera by four species viz., Macrothrix species, Alona rectangula, Chydorus sphaericus and Alonella species, Protozoa, Copepoda and Ostracoda each by only one species i.e. Centropyxis, Mesocyclops and Stenocypris respectively and a larval form i.e. Nauplius larva.
Rotifer fauna in presently studied fish pond showed two peaks quantitatively i.e. one during the month of May (summer) and other during July (monsoon) and remains totally absent in the months of September and February. Both quantitatively and quantitatively rotifers showed increasing trend from spring to monsoon (Fig 1a,b,c,d,e and Fig 2a,b,c,d,e) which may appear to be favoured by increased photoperiod and high temperature (Kumar, 1991). Annual maxima of rotifers during monsoon (July) may be correlated with detritus enrichment, resulting from catchments, along with monsoon rains. Jyoti and Sehgal (1979) found maximum rotifer diversity in July and August from littoral and in August from limnetic zone of lake Surinsar while Kumar et al.(1991) recorded rotifer’s quantitative peak during June, August and January. On the basis of presence of Brachionus species, Keratella species and Filinia species, the present water body can be categorized as mesotrophic to eutrophic (Pejlar, 1957).
A perusal of table 1 shows that cladocerans were represented by 4 genera and they showed peak during the month of February which was contributed by single species i.e. Chydorus sphaericus which may be attributed to lower temperature. Similar observations showing cladoceran peak during winter have been reported by Khan and Sidique (1974), Kalk (1979), Sehgal (1980), Saint- Jean (1983) and Sanjer and Sharma (1995). Minimum number of cladoceran count was observed during monsoon months. This decreased count may be attributed to dilution. Shadin(1962) suggested that under turbid condition, silt accommodate in their digestive tract and thus resulting their sinking to bottom.
Centropyxis aculeate is the only protozoan recovered from pond and its population acquire maxima in the month of August i.e. 26 n/l (Table1). Whereas during the months of March, May, September and October it remains totally absent. The growth in protozoan population during monsoon (August) is related to availability of organic matter and detritus on which these organisms feed (Sorokin and Paveljeva, 1972 and Kumar, 1990).
Copepods qualitatively represented by one species and one of its larval form i.e. Nauplius and quantitatively outnumber all other plankter during spring (April) and winter (January). Mesocyclops remained dominant and showed its presence at both low and high temperature thus suggesting its facultative / eurythermic nature.
Ostracoda was represented by only one species i.e. Stenocypris species during the period of present investigations. Ostracoda dominated over other groups during autumn which may be attributed to the favourable thermal and related limnological conditions.
On the basis of the present limnological survey of zooplankton, it was revealed that following zooplanktivorous fishes like Catla catla, Cirrhina reba, Hypopthalmixthys molitrix (silver carp) etc. which are of economic importance could be reared in presently investigated fish pond so as to ensure maximum utilization of live food.
References
- A.P.H.A., Standard method for the examination of water and waste water. Washington. D.C. 1193PP (1976).
- Adoni,A.D., Workbook on limnology.Pratibha Publishers, C-10 Gour Nagar, Sagar, India (1985).
- Edmondson, W.T. and Winberg, C.G., A manual on methods for assessment of secondary production in freshwater.IBP Hand book No.17: 358 (1971).
- Gaur, R.K., Khan, A.A., Alam, M.A. and Alam, A., Pollution status of a Leachate reservoir receiving effluents from thermal power plant with reference to plankton population.In: “Freshwater Ecosystem of India”.Ed: K.Vijay Kumar,(1999) 226-236.
- Kalk, M., Zooplanktons of lake Chilwa: Adaptation to change in: J. Illies (Ed.) Lake Chilwa: Monographiae Biologicae, (1979) 35: 123-141.
- Khan, A.A. and Siddiqui, A.Q., Seasonal variation in the limnology of a perennial pond at Aligarh. India. J. Fish, (1974) 21 (2):154-174.
- Kumar, A. and Singh,A.K., Role of rotifers as bioindicators in freshwater lentic ecosystems. In.”Freshwater Ecosystem of India”.Ed: K.Vijay Kumar,173-186 (1999).
- Kumar,N., Altitudes related limnological variations in some fish ponds of Jammu Province. Ph.D.Thesis,University of Jammu (1990).
- Kumar,N.,Dutta,S.P.S.,Malhotra,Y.R.and Kumari,V., An ecological study of rotifers in Kunjwani Pond Jammu. J. Hydrobiol. (1991) 7: 41-45.
- Nikolsky,G.V., Ecology of fishes,Academic Press London and N.Y (1963).
- Pennak,R.W., Field and experimental limnology of three Colorado mountain lakes. Ecology. (1968) 19(3): 505-20.
- Sain-Jean,L., The Zooplankton In: J.P. Carmouse et al., (Ed) Lake Chad. Monographiae Biologicae, (1983) 199-232.
- Sanjer, L.R. and Sharma, U.P., Community structure of plankton and their periodicity in Kawar lake: Begusarai, Bihar. J. Freshwater Biology. (1995) 7 (1): 33-36.
- Sehgal, H.S., Limnology of lake Surinsar, Jammu with reference to zooplankton and fishery prospects. Ph.D.Thesis, University of Jammu. Jammu (1980).
- Shadin, V.P., Wechsel der Biozonose beim Ubergang von Fluss Zu Stansee. Schweiz. Z. Hydrobiol. (1962) 24: 459-466.
- Sorokin Y.I. and Paveljeva,E.B., On the quantitative characteristic of the pelagic ecosystem of Dalnee Lake (Kamchakta). Hydrobiol. (1972) 40: 519-552.






