Ferronia elefuntum fruit shell : A carrier for the removal of Pb (II) from aqueous solution
U.E. Chaudhari1 *
1
Department of Chemistry,
Mahatma Fule Arts, Commerce and Sitramji Chaudhari Science College,
India
DOI: http://dx.doi.org/10.12944/CWE.4.2.20
The studies on removal of Lead (II) were conducted using Ferronia Elefuntum Fruit shell. Adsorption efficiency has been evaluated. The effect of pH, contact time, adsorbent dose, concentration of metal, particle size and temperature were studied. The results reveal that Langmuir and Freundlich isotherms are followed during adsorption process. Thermodynamids parameters indicate the feasibility of the process. Kinetic studies have been performed to understand the mechanism of adsorption. Column studies have been carried out to compare these with batch capacities.
Copy the following to cite this article:
Chaudhari U.E. Ferronia elefuntum fruit shell : A carrier for the removal of Pb (II) from aqueous solution. Curr World Environ 2009;4(2):393-397 DOI:http://dx.doi.org/10.12944/CWE.4.2.20
Copy the following to cite this URL:
Chaudhari U.E. Ferronia elefuntum fruit shell : A carrier for the removal of Pb (II) from aqueous solution. Curr World Environ 2009;4(2):393-397. Available from: http://www.cwejournal.org/?p=994
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-08-22 |
|---|---|
| Accepted: | 2009-09-30 |
Introduction
The Twentieth century star ted with an extensive damage to the natural resources¹. Unplanned industrialization, urbanization, pollution explosion, change in life-style, over exploitation of natural resources, commercial establishment and modern agricultural practices have degraded the quality of environment. The main effects being faced are:
• Continental invasion of air and water.
• Marine pollution through waste discharges.
• Release of variety of chemical and biological contaminates into the water bodies, on land and in air.
• Ground water pollution.
• Acid rains and nuclear fallout.
These effects are not only covering the pollution of environment but also are responsible in creating genetic erosion in plants, animals including human beings and microorganisms. Water is a prime natural resource and is a basic human need. The availability of adequate water supply in terms of its quality and quality is essential for the existence of life.
Water is available in nature as surface water and ground water through the self-purification mechanisms like physical, chemical and microbiological processes at natural bodies are carried out in nature. However, natural water is rarely suitable for direct consumption to human beings. Rapid industrialization and population growth resulted to generation of large quantities of wastewater and causing problem of their disposal. Industrial waste constitutes the major source of various kinds of metal pollution in natural water. The presence of heavy metals in the environmental has been of great concern because of their increased discharge, toxic nature and other adverse effects on the receiving streams. When the concentration of toxic metal ions exceed tolerance limit, they may become real health concern². There is an immediate need to introduce cleaner technologies to minimise the pollution and to protect the degrading environment. It is not possible to achieve zero waste discharge, but it is an essential to treat the waste.
Among the toxic heavy metal ion which present in potential health hazard to aquatic animals and human like Pb, Cd, Cr, V Bi & Mn are important.
The maximum tolerance limit for Lead (II) for public water supply are 0.1mg/L. Toxicity of metal depends on the type of metal, dose and the ionic fo r m . Lead is extensively used in pr inting, manufacture of paints, water pipes, storage battery manufacture, pottery and soldering operations etc. Besides it is used as a antiknock agent in gasoline. Toxicity of Lead3 include anemia, acute poisoning like acute abdominal colic and syndrome of acute encephalopathy. It causes mental deterioration convulsive seizures, severe central nervous system depression and death.
Literature survey reveals that, there are many methods namely coagulation, precipitation, ion exchange and adsorption, for removal of Lead (II) metal ions from aqueous medium. However, adsorption is an easy and economical process for removal and retrieval of cation from aqueous medium. Efficiency of adsorption process mainly depends on nature of adsorbent, adsorbate, pH, concentration, temperature, time of agitation etc.
The cheap and efficient adsorbents can carry to cater the need of population in the rural areas and the population in the industrial area where safe drinking water is not available. In the present study, Lead (II) is removed by using Ferronia elefuntum Fruit [4 to 8] as a adsorbent.
Adsorbent
The Ferronia elefuntum Fruit Shell was first dried at temperature of 160°C for 6 hours. After grinding it was sieved to obtain average particle size of 200 mesh. It was then washed several times with distilled water to remove dust and other impurities. Finally it was dried again in an oven at 50°C for 6 hours. The adsorbent was then stored in desiccator for final studies.
Batch Study
The dried amount of 0.5 gms of Ferronia elefuntum Fruit Shell was taken in 250 ml reagent bottle and synthetic solution (200 ml) containing various concentration of Lead (II) ion was added and system is equilibrated by shaking the contents of the flasks at room temperature so that adequate time of contact between adsorbent and final concentration of metal ion. Lead (II) was determined by spectrophotometry9 using Dithizone method at 515 nm against a reagent blank. The spectrophotometer, systronic model 104) was used to measure the concentration of Lead (II) ions.
Equilibrium adsorption isotherm for Ce verses qe, plotted for Ferronia elefuntum Fruit Shell are shown in figure 1. The adsorption capacity in mg/L was calculated then the equation. Qo = (Co-Ce) V/M where, Co is the initial concentration of Lead (II) Ce is the concentration of Lead (II) at equilibrium in mg/L V is the volume of solution in litre and M is the mass of adsorbent in grams
Adsorption Isotherms
Equilibrium isotherms was studied for both Langmuir and Freundlich isotherms. The results are shown in Fig. 2 and 3 which illustrate the plot of Langmuir and Freundlich isotherms of Ferronia elefuntum Fruit Shell for Lead (II). The saturated monolayer can be represented by: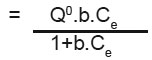 The linearised from of the Langmuir isotherm is
The linearised from of the Langmuir isotherm is 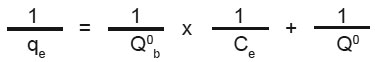 Where, Q0 and b are Langmuir constants.
Where, Q0 and b are Langmuir constants.
The plot of 1/Ce Vs 1/qe was found to be linear, indicating the applicability of Langmuir model. The parameters Ï•0 and b have been calculated and presented in Table 1. The Langmuir constant Q is a Measure of adsor ption capacity and b is a measure of energy of adsorption. In order to observe whether the adsorption is a favourable or not, a dimensionless parameter 'R' obtained from Langmuir isotherm is. R = (1 + b x Cm)-1 where, b is Langmuir constant and Cm is maximum concentration used in the Langmuir isotherm.
The adsorption of Lead (II) on Ferronia elefuntum fruit shell is a favourable process as 'R' values lie between zero to one. Coefficients of co- relation (r) are also shown in Table 1. The applicability of Freundlich isotherm was also tried using the following general equation: qe = k.CeB linearised form of this equation is log qe = B.log Ce + log k. Where, B and k are Freundlich constants.
These constants represent the adsorption capacity and the adsorption intensity respectively. Plot of log qe Vs log Ce was also found to be linear. The values of B and k are presented in Table 1. Since the values of B are less than 1, it indicates favourable adsorption.
 |
Table 1: Isothermal constants Click here to view table |
Results and Discussion
The response of Adsorbate dose and contact time on the removal of Lead (II) is presented in figure (I). The observations reveal that an increase in the adsorbate dose, rate of adsorption increase upto certain level and then it become constant. Also as the time of contact increase, adsorption increase and them it become constants.
Effect of pH on the Removal of Manganese (II)
The effect of pH on the removal of Manganese (II) is shown in Fig. 4. Experiment were conducted at the constant initial Lead (II) concentration, adsorbent dose (Ferronia elefuntum fruit shell) of 0.5 gm/100 ml and the contact time of 4 hours. The pH of the aqueous solution is an important controlling parameter in the adsorption process. It was observed that the percentage removal of Lead (II) is higher at pH = 5 and then decrease with increase of pH.
Effect of Particle Size
The adsorbent particle size has significant influence on the kinetics of adsorption. The influence of particle size furnishes important information for achieving optimum utilisation of adsorbent. Four particle size 50, 100, 150, 200 micron size (Indian Standard Sieves) under optimum condition. It is found that, as the particle size increase the rate of adsorption decrease.
Kinetics of Adsorption
0.5 gm of Ferronia elefuntum fruit shell and 200 ml Pb++ solution was taken in 1000 ml R. B. and shake vigoriously for about four hours. After every 15 minutes, 5 ml sample of the solution was withdrawn for the first hour and subsequently the interval between the samples withdrawn was increased to 30 minutes. The concentration of the metal ions in the sample, withdrawn were determined by the spectrophotometry and were designated as C and the value of the concentration of the metal ion on the Ferronia elefuntum Fruit Shell at the same time interval estimated using the relation. q = (Co - Ct) V/W
The rate of adsor ption of Lead(II) of Ferronia elefuntum fruit shell was studied by using the first order rate equation proposed by Lagergren10 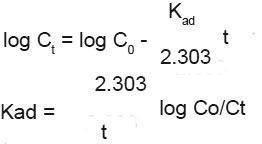 Where Kad is the rate constant for adsorption. The plote of log Ct Vs t is shown in Fig. 5.
Where Kad is the rate constant for adsorption. The plote of log Ct Vs t is shown in Fig. 5.
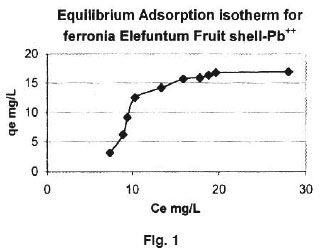 |
Figure 1 Click here to view figure |
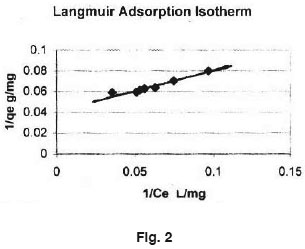 |
Figure 2 Click here to view figure |
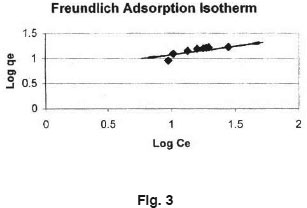 |
Figure 3 Click here to view figure |
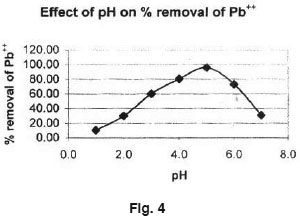 |
Figure 4 Click here to view figure |
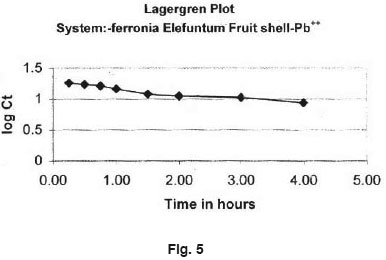 |
Figure 5 Click here to view figure |
Conclusions
- The percentage retrieval of Lead (II) is formed to be increase with decrease the initial concentration of Lead (II). The removal is found rapid in initial stages followed by slow adsorption upto saturation limit.
- The developed technique of retrieval of Lead (II) ions using Ferronia elefuntum Fruit Shell appears to be a cheap and practically viable for the use of semiskilled workers in villages.
- The present work on adsorption process is in good agreement with Langmuir isotherm indicating monolayer adsorption process.
- The results on adsorption process reveals that at pH = 5.0, Lead (II) uptake capacity is better.
- The straight lines plots of log C versus time for the adsor ption show the validity of Lagergren equation and suggest the first order kinetics.
- Regeneration studies are not necessary with the view that the cost of the adsorption is very and it can be disposed of safely.
References
- Mamta Tomer, "Quality Assessment of water and Wastewater", Lewis Publisher Boca Ratan, (1999).
- Singh D. K. and Lla Jyosna, "Removal of toxic heavy metal ion from wastewater by coal based adsorbent", Pollution Research,(1992) 11: 37-42.
- Chandra S. V., "Toxic Metals in Environment, "Published by Industrial toxicology research centre Luckhnow.
- B. D. Gharde, S. B. Gholese, P. V. Patil, "Removal of Cu (II) and Ni(II) from solution using Ferronia elefuntum Fr uit Shell, proceeding of NSAM,(2004) 166-119.
- S. B. Shukla, V.D. Sakhardande. Column studies on metal ions removal by dyed cellulosic. Material J. Appli polym. Science,(1992) 44(5), 903-10.
- V. W. Lhangan, D. B. Bnakar and S.S. Dara effectiveness of terminalia bellerica bark for scavenging zinc ions. Chem. Environment Res.,(1992) 1(1): 87-94.
- P. R. Rampure and P. V. Patil. Use of modified Dhoda Bark for scavenging Cd ions from Industrial waste-water Jr. of Industr ial pollution control (1996) 12(1).
- P. R. Rampure and P.V. Patil. Use of palsa Bark substrate for the recovery of Cu, Pb, Zn, Ni from waste-water Jr. of Industrial pollution control (1996) 12(1).
- Vogel, A. L. "Textbook of Quantitative Inorganic Analysis", Forth Edition,(1978) 735-736.
- Lagergren, S and Bil K, Svenska Vatenskapsakad hand (1998) 24.







