Adsorption removal of pollutants (triphenylmethane and xanthene dyes) from water by charfines and activated carbon
K.N. Singh1 * and Shashi Kant Rai1
1
Department of Chemistry,
S.G.R.P.G.College,
Dobhi,
Jaunpur,
222 149
India
DOI: http://dx.doi.org/10.12944/CWE.5.2.20
The adsorption removal of Triphenylmethane & Xanthene dyes onto a low-cost coal based adsorption (Charfines) and its efficiency in dye colour sorption was compared with activated carbon (F-400). Batch studies were performed and the results revealed that Charfines demonstrated on ability to adsorb the reactive Triphenylmethane & Xanthene dyes. The sorption interaction of reactive dye on to Charfines obeys the first order rate equation. The sorption data indicates that the adsorptive removal of the dye from equeous solution is rather comlex involving both boundary layer diffusion and interporticle diffusion. The process of dye sorption while activated carbon resulted in physisorption interaction. Dye sorption is found to be dependent on the aqueous phase pH and the dye uptake is greater at lower pH.
Copy the following to cite this article:
Singh K. N, Rai S. K. Adsorption removal of pollutants (triphenylmethane and xanthene dyes) from water by charfines and activated carbon. Curr World Environ 2010;5(2):345-349 DOI:http://dx.doi.org/10.12944/CWE.5.2.20
Copy the following to cite this URL:
Singh K. N, Rai S. K. Adsorption removal of pollutants (triphenylmethane and xanthene dyes) from water by charfines and activated carbon. Curr World Environ 2010;5(2):345-349. Available from: http://www.cwejournal.org/?p=1219
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-10-14 |
|---|---|
| Accepted: | 2010-11-17 |
Introduction
Dyes are synthetic aromatic compound, the textile industry ranks first in dye consumption for the coloration of fabric, presently more than 9000 distinetly different dyes are listed in the colour index dyes are classified into various application classes (Direct, Acid, Basic, Reactive etc.) and chemical class (Azo, Xanthane, Triphenylmethane,Thazine etc.). The dye stuffs are water soluble dispersible organic colourans,having high porential use in various industrial applications(mostely in the textile and paper/leather industry as a colouring material).1 Most of the aromatic dyes are fused with inorganic metals. According to an estimate around 10% of the dye used in production processes enter as waste into the environment through effluent.3 Among the dyes, the Triphenylmethane & Xanthene group of dyes are the largest and most versatile class of dyes and more than half of the annually produced dyes are Triphenylmethane & Xanthene dyes.5
The treatment of dyes-bearing effluent is one of the major problems faced by the industries and the recent stringent regulation exacerbate the problems further, recently, same countries have banned the use of the xanthenes dyes. Various physical, physico-chemical, biological and chemical processes have been investigated for dyes colour removal6. Biological processes are seldom capable of removing the dye colour because of its nonbiodegradable nature.6-9 Chemical processes like coagulation and adsorption appear to be highly effective in removing dye colour from the effluent. Among various treatment technologies adsorption onto activated carbon has proved to be one of the effective and reliable physicochemical treatment methodologies.7,8,10 However, the cost of activated carbon and regeneration problems necessitated the search for other low cost adsorbent. In this context, various material like inorganic clay materials industrial waste (by-product) water etc, have been widely studied.6,8,9,11
In this contribution, we present the results obtained from experimental investigation performed with a low cost adsorbent (Charfine-a by product obtained during carbonization of lignitc coal) in the process of adsorptive colour removal of a reactive dye belonging to the Triphenylmethane & Xanthene chemical class and results were compared with activated carbon.
Experimental
Adsorbent Charfines
A by product in the carbonization processes of lignite coal was acquired from the water pollutant (industries) and used as the adsorbent for removing dye colour from the aqueous phase, Charfines initially cleaned and sieved to a geometric mean(gm) 106um was used as an adsorbent in the study. Elemental analysis performed on Charfines revealed it to have carbon 62.8%, Hydrogen 1.47%, Nitrogen 0.84% and Oxygen 17.4%, Activated carbon was used as a refere adsorbent to assess the potential of the Charfines as an adsorbent in the process of dye colour removal. Xanthene dye belonging to the reactive application class was used as the test dye. The required quantity of reactive red dye was accurately weighed and dissolved in distilled water to prepare the stock solution. The solution of the required concentration were prepared by successive dilution of the stock solution.
Adsorption Studies
All the batch tests were performed at room temp.(27±2°C ),the reaction mixture consisting of 50ml of 50mg/l reactive dye solution and 200mg Charfines was taken in a 250ml stoppered glass bottle and agitated on a horizontal shaker at a rate of 100rpm to over come diffusion resistance to the boundary layer during sorption process. At the end of the desired contact time ,the bottle were taken from the shaker, the contents filtered and analysed for residual colour concentration. Kinetics of sorption reaction to determine the equilibrium time of dye sorption were performed by analysing the residul colour concentration at predetermined time of 10,20,40,60,120,240 minutes and the results was used for the subsequent batch experiment.The effect of pH on the sorption was studied by conducting equilibrium test of different pH value of the solution ranging from 2-11. The pH of the reaction mixture was adjusted using 0.1N H2SO4 and 0.1N NaOH. Isothermal studies were conducted by varying the dose of adsorbent and by keeping the concentration of the adsorbate constant, adsorbing the colouring substance onto Charfines, separating the adsorbent and then introducimg it into the bottle containing distilled water, organic and inorganic solvents, agitated for one hour and analysed for colour concentaration.
Results and Discussion
Kinetic Study
The rate of dye colour uptake by activated carbon (F-400) and Charfines at different contact period is shown in fig 1. In the case of Charfine, it is shown that the initial rate of dye uptake was rapid and it gradually attained equilibrium. In which the first inital peak portion is indicative of high adsorption uptake of the dye molecule. This may be attributed to the chemisorptions type of interaction. The second phase indicates the slow uptake of dye molecule which indicates the utilization of all active sites over the adsorbent surface and attainment of the saturation or equilibrium phase. The third phase is the equilibrium phase in which the sorption uptake is relatively negligible.The initial high uptake of the dye at relatively less contact interaction of dye molecule over the Charfines. This type of sorption is reported in the case of dye sorption on different adsorbent.6,8,9,10,13
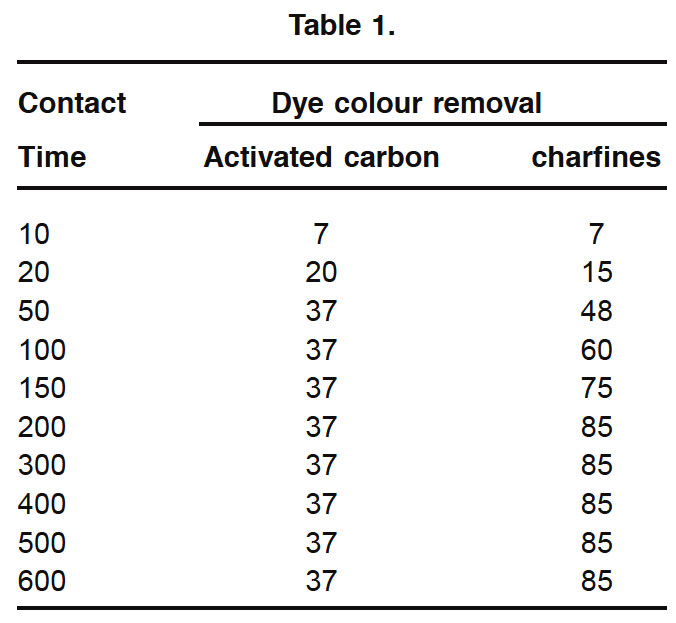 |
Table 1. Click here to View table |
 |
Table 2 |
In the case of activated carbon, the shape of the uptake plot was some whate different to that of Charfines. The initial dye uptake was slow and gradually attained equilibrium. This may be due to the fact that the activated carbon is of micro and the dye uptake will have to first encounter the boundary layer it has to diffuse onto the adsorbent surface and finally it has to diffuse into the porous structure of the adsorbent.
Sorption rate study
Contact time experiment which describe the rate of uptake of the adsorbate by the adsorbent may be used to establish the dynamic of sorption reaction in terms of the order of the rate constant of the reaction. The first order rate constant by assuming sorption interaction of the dye over studied adsorbent as a first order reaction. The first order rate equation – log (C/Ce) = (K/2.303) *t
Where C is the initial dye colour concentration (mg/l), Ce is equilibrium dye colour concentration (mg/l), t is contact time (min) and K is first order reaction rate constant (min).
 |
Figure 1: Effect of contact time on reactive dye sorption (mass of the sorbent = 1000mg, Initial dye concentration =50mg/l, temp. 27±2°C Click here to View figure |
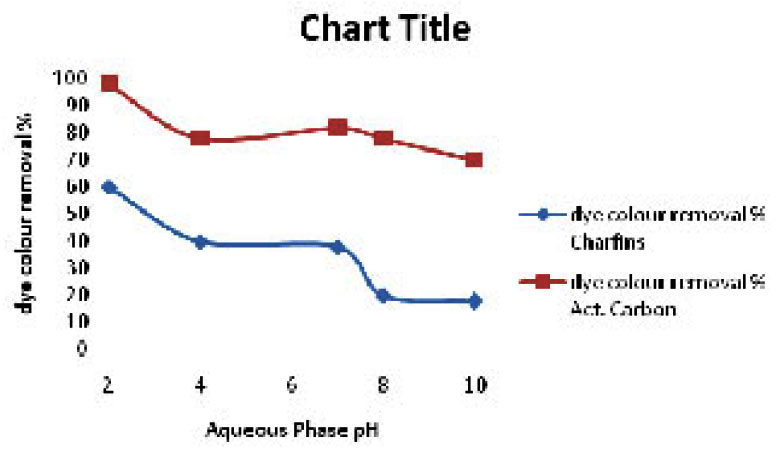 |
Figure 2: Effect of solution pH on dye sorption (mass of the sorption = 1000mg, Initial dye concentration = 50mg/l,Temp. 27±2°C Click here to View figure |
The above equation is y = mx type of a linear plot of log (C/Ce) versus t indicate the validity of the first order reaction. The values of the rate constant are obtained from the slope of the straight line of the plats fig2 as 0.0091 min and 0.0074 min for Charfines and activated carbon respectively. The straight line of the plot indicate the validity of the first order rate reaction enumerating the dye sorption interaction onto the Charfines and activated carbon is of the first order.
Effect of the aqueous phase pH on dye solution
The pH of the aqueous medium exerts a profound influence on the sorptive uptake of the dye colour presumably due to its influence on the surface of the sorbent and ionization of the molecule. The effect of the pH of the solution on the adsorption uptake by Charfines and activated carbon is shown in fig.3. The dye uptake was higher at lower pH and as the pH of the dye solution increased, dye uptake decreased considerably. Studies to investigation the influence of the pH on adsorption uptake indicate that the maximum uptake of the dye occurred in the pH range of 2.0 to 6.0. At lower pH, the surface of the adsorbent becomes positively charged and this would facilitate sorption of the colour cation probably by exchange sorption. The low sorption uptake at a higher pH range may be due to competitive adsorption of OH ions and dye anion.1,9,12
Conclusions
Adsorption studies performed on the low cost coal based adsorbent (Charfines- a by product obtained during corbonization of lignite coal) revealed its ability to remove reactive xanthane dye from the aqueous phase. Batch kinetic studies performed on the coal-dye system indicated the varied dye sorption capacity of the tested adsorbent (Charfines and activated carbon). The initial high uptake of dye on Charfines may be reasoned to be due to chemisorptions interaction while gradual uptake by activated carbon may be indicative interaction. Coal-dye interaction concurs with the first order rate equation and intrapartical diffusion studay indicating that intraparticle diffusion is a complex interaction of the boundary layer effect.
Acknowledgements
The authors are thankful to Dr. Rakesh Singh, Principal S.G.R.P.G.College, Dobhi, Jaunpur for providing all necessary experiment facilities for performing our research work.
References
- Alexander F, Mckay G., Kinetics of the removal of basic dye from effluent using silica. The chemical engineer (1997) 319: 43-247.
- Brown D, Anliker R., In : Richardson ML(ed) Risk assessment of chemical in the environment, Royal Society of Chemistry, London (1988).
- Clorke D, Anliker R, In : Hutzinger O(ed) The Handbook of Enviromental Chemistry vol.3A, Springer-veriag, Berlin Heidelberg, New York (1980).
- Ojha, Priyanka,Investigation of the Removal of Methylene blue and Metomega cheome orange dyes by Rice husk, Coal and Graphite surface, Ph.D. Thesis V.B.S.Purvanchal University,Jaunpur, India (2008).
- Stolz A., Basic and applied aspects in the microbial degradation of Azo dyes,Appl microbial Biotechnol (2001) 56: 69-80
- Karthikeyan J., Removal of colour from industrial effluents, In : Trivedi RK(ed) Pollution management in industries. Environmental Publication, Karda (1988)
- Venkata Mohan S, Karthikeyan J, Removal of Lignin and Tannin colour from aqueous solution by adsorption onto Activated charcoal. Environmental poll. (1997) 97: 183-187.
- Venkata Mohan S., Removal of Taxtile dye colour from aqueous solution by adsorption onto Coal based sorbents. Ph.D. Thesis, S.V.University, Tirupati (1997).
- Venkata Mohan S, Chandrasekhar Rao N, Singh et al., Curr. World Environ., Vol. 5(2), 345-349 (2010) 349 Karthikeyan J., Adsorption removal of direct azo dye from aqueous phase onto coal based sorbents: a kinetic and mechanistic studay.J Hazardous Materials (2002a) 90(2): 189-204.
- Karthikeyan J, Chaudhari M., Echancement of mercury(II) sorption from water by coal through chemical pretreatment. Wat Res., (1986) 20: 449-452.
- Namasivayam C, Yamuna RT, Removal of Cango Red from aqueous solutions by biogas waste slurry, J Chem Tech Biotechnol (1992) 53: 153-157.
- Venkata Mohan S, Chandrasekhar Rao N, Krishan Prasad K, Treatment of Simulated Reactive yellow 22(azo) dye effluents using spirogyra sp.waste. Manag (2002b) 22: 575-582.
- Ahmed MN, Ram RN, Removal of basic dye from waste-water using silica adsorbent. Env. Poll. (1992) 77: 79-86.







