Clean Environment-Clean Technologies, Hydrogen Peroxide for Clean Environment
Alina Jyothi Joseph1 * , Kalpana Madgula1 and Rita Kakkar1
DOI: http://dx.doi.org/10.12944/CWE.6.1.17
If any substance is interesting, it’s hydrogen peroxide. By now everyone’s aware of the ozone layer that surrounds the earth. Ozone consists of three atoms of oxygen (03). This protective layer of ozone is created when ultraviolet light from the sun splits an atmospheric oxygen molecule (02) into two single, unstable oxygen atoms. These single molecules combine with others to form ozone (03). Ozone isn’t very stable. In fact, it will quickly give up that extra atom of oxygen to falling rainwater to form hydrogen peroxide (H202). It is this hydrogen peroxide in rainwater that makes it so much more effective than tap water when given to plants. With the increased levels of atmospheric pollution, however, greater amounts of H202 react with air-borne toxins and never reach the ground. To compensate for this, many farmers have been increasing crop yields by spraying them with diluted hydrogen peroxide. We can achieve the same beneficial effect with house plants. If you’ve never used Hydrogen Peroxide you are overlooking one of the most powerful healing tools ever discovered. Most of us started on hydrogen peroxide shortly after birth. Not only does mother’s milk contain high amounts of H202, the amount contained in the first milk (colostrum) is even higher. This seems only reasonable now that we know one of its main functions is to activate and stimulate the immune system. Hydrogen peroxide is safe, readily available and dirt cheap. And best of all, it works! We do know that it is loaded with oxygen. (Half a liter of the food-grade 35% solution contains the equivalent of 65 lits of oxygen under normal conditions. We also know that when H202 is taken into the body (orally or intravenously) the oxygen content of the blood and body tissues increases dramatically. Hydrogen Peroxide is most versatile chemical used in various industries as bleaching agent, reagent in chemical synthesis, environmental control / effluent treatment, sterilization etc. The important constituent being active oxygen which is obtained by the controlled decomposition of H2O2 and water as a by-product. In this paper, the usage of H2O2 to provide ‘clean’ processes, without the production of any harmful or environmentally unsafe product is presented.
Copy the following to cite this article:
Joseph A.J, Madgula K, Kakkar R. Clean Environment-Clean Technologies,Hydrogen Peroxide for Clean Environment. Curr World Environ 2011:6(1);125-130 DOI:http://dx.doi.org/10.12944/CWE.6.1.17
Copy the following to cite this URL:
Joseph A.J, Madgula K, Kakkar R. Clean Environment-Clean Technologies,Hydrogen Peroxide for Clean Environment. Curr World Environ 2011:6(1);125-130. Available from: http://www.cwejournal.org/?p=1297
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-04-01 |
|---|---|
| Accepted: | 2011-05-08 |
Introduction
Hydrogen peroxide is a very strong oxidizer and it burns any organic matter in contact. For instance, if you soak a cotton rag with 90% hydrogen peroxide it burns very quickly. It can react in a hypergolic way if mixed with other chemicals. Thus dilution of H2O2 should be monitored according to the application it is used for H2O2 is used in Industrial scale for the production of organic and inorganic chemicals, as a bleaching agent in Textiles, paper pulp and non-edible oil production, as a detoxifier for industrial waste water. The versatility is further enhanced by properties like: Effective over whole pH range, high oxidation potential, non contaminating by-products, is a liquid and so, easy to use.
Hydrogen Peroxide – Aerobic and Anaerobic Bacteria
The friendly bacteria are aerobic. In other words, they flourish in high oxygen environments and thrive in the presence of oxygen rich H202. On the other hand, most strains of harmful bacteria are anaerobic and cannot survive in the presence of oxygen or H202. We can agree that hydrogen peroxide produced within individual body cells is essential for life. And no one doubts its effectiveness when it comes to even treating infections topically.
In BOD/COD Removal as an Oxygen Supplemental Source
Hydrogen peroxide has been used to reduce the BOD and COD of industrial wastewaters for many years. While the cost of removing BOD/ COD through chemical oxidation is typically greater than that through physical or biological means, there are nonetheless situations which justify its use. H2O2 can be used as a stand alone treatment or as an enhancement to existing physical or biological treatment processes, depending on the situation. H2O2 can be used alone or with catalysts such as iron, UV light, ozone (O3) and alkali to oxidize BOD/ COD contributing compounds in wastewaters.
2H2O2 → 2H2O + O2
If a large fraction of the BOD/COD is contributed by inorganic reduced sulfur compounds such as sulfides or thiosulfate, then H2O2 alone is typically effective. Depending on the wastewater pH, the oxidation of these compounds by H2O2 yield sulfate or colloidal sulfur, neither of which contribute to BOD/COD. If the primary contributors to BOD/ COD are dissolved organics, then a more reactive oxidation system is needed. A generalized reaction for reducing BOD/COD with Fe+2 as catalyst can be expressed as follows:
BOD/COD + H2O2 → partially oxidized species → CO2 + H2O + inorganic salts
When H2O2 is used to supplement DO, it is metered directly into the aeration basin of a biological treatment system to provide an immediate source of DO. The conversion of H2O2 to DO in an activated sludge mixed liquor proceeds according to the following reaction:
(Catalase Enzyme)
2 H2O2 → O2 + 2 H2O
Theoretical H2O2 requirement: 0.48 lbs H2O2 (100%) per mg/L DO. The above reaction shows that two parts of H2O2will yield one part of DO. Therefore, the amount of H2O2 required to oxygenate the wastewater is surprisingly small.
Aquaculture in India
India is making rapid strides with its Blue revolution and today, ranks third in the world in aquaculture. Prawns and shrimps rank as the highest foreign exchange earner among our marine product exports, with framed shrimps accounting for close to half of the total shrimp exports in volumes and fetching over 70 per cent in value.
India is a major supplier of shrimp to Japan, Europe, USA and still has tremendous untapped potential in this segment. Improved yields and adherence to stringent environmental regulations like HACCP (Hazard Analysis Critical Control Point), International Food Quality Standards etc., imposed by international bodies like the European Union and US FDA play a pivotal role in enhancing business prospects for export of Indian aquaculture products. In order to meet these standards, it is necessary that we have to grow Contamination and Disease free Aquaculture products. Pond parameters such as water hardness, acidity/ alkalinity, dissolved oxygen levels play an important role in development of a good culture harvest. Marine products produced in culture ponds are also prone to infections by microorganisms, including fungal, bacterial and viral infections.
Hydrogen Peroxide in Aquaculture
Oxygen is one of the critical factors for the aquatic life, especially for shrimps and scampi. Though some amount of Dissolved Oxygen (DO) is always there in the ponds, under semi-intensive shrimp farming the rate of depletion of DO is quite high, but such drops make the culture weak, susceptible to diseases and reduce their growth rate. On the other hand, such conditions favor the growth of harmful bacteria and promote the anaerobic decomposition of feed and other organic matter including culture excreta and wastes leading to production of toxic materials, besides sludge formation. This further affects the health, productivity and survivability of the culture.
Hydrogen Peroxide brings the assurance of a new life for farms and ponds. Hydrogen Peroxide generates and releases the lifesaving oxygen and helps maintain the Dissolved Oxygen levels of the pond. Reduce the sludge formation and reduce the chances of Bacterial and viral diseases. Also used to lower the organic load in ponds and helps in inducing moulting in prawns. Hydrogen Peroxide will be very useful in increasing the Dissolved Oxygen levels, especially during like rainy days, cloudy weather, high bacterial count in water and summer when oxygen levels decrease in the ponds. This is mainly used for prawn culture as the prawns crawl at the lower surface of pond unlike fishes that move on the top layer of water and consume oxygen from air.
Dosage
3-5 kgs of Hydrogen Peroxide (30%) per Hectare of pond.
Hydrogen Peroxide as Dechlorinating Agent
Hydrogen peroxide (H2O2) is a safe, convenient alternative for many dechlorination needs, especially those involving “free available chlorine” as opposed to “combined available chlorine.” The chlorine as HOCl and OCl-is referred to as free available chlorine. This is the form of chlorine typically found in cooling water circuits, industrial bleaching systems, and many chemical processing operations. Nitrogen containing compounds such as ammonia, amines and proteins are usually present in municipal wastewater. Free available chlorine reacts readily with these materials to form chloramines in which the chlorine is described as combined available chlorine. The available chlorine remaining after disinfection of municipal wastewaters is usually present in the combined form. Dechlorination can be accomplished by several means, the most widely used being sulfur dioxide – either as a gas (SO2) or as a salt (e.g., sodium metabisulfite). However, this method has several short comings. Hydrogen peroxide reacts with free available chlorine in solutions with pH > 7. While there is no upper limit to the pH (e.g., H2O2 can be used to dechlorinate effluent from caustic/chlorine odor scrubbers), as a practical matter, pH 8.5 is preferred in order to provide an instantaneous reaction.
Cl2 + H2O2 →O2 + 2HCl
About 200 gms of hydrogen peroxide is required to destroy 500 gms of free available chlorine. In most cases the oxygen produced by the reaction will remain dissolved in the solution (saturation is about 10 ppm D.O.). Where higher concentrations of chlorine are involved, the solutions may effervesce and provision must be made to accommodate the O2 evolved. The reaction is mildly exothermic, liberating 37 kcal/mole as opposed to 199 kcal/mole when using SO2. Significantly, hydrogen peroxide reacts very slowly with combined available chlorine. Consequently, solutions which contain ammonia (e.g., most municipal wastewater effluents) cannot be dechlorinated with H2O2.
Hydrogen peroxide should be investigated as a dechlorination agent in industrial waters characterized by free available chlorine. These include: Cooling water blowdown where chlorination is used for microbiological control. Municipal wastewater effluent that has been denitrified prior to chlorination. Tests show 100% fish survival after 96 hours in the undiluted hydrogen peroxide-treated effluent. In fact, the dissolved oxygen from the hydrogen peroxide reaction with chlorine may improve the quality of the receiving water.
As a Disinfectant
Among other applications, hydrogen peroxide is used as a disinfectant. It is used to treat inflammation of the gums and to disinfect (drinking) water.
In the United States, hydrogen peroxide is used more and more frequently to treat individual water supplies. It is used to prevent the formation of colors, tastes, corrosion and scaling by pollution degradation (iron, manganese, sulphates) and micro-organism degradation. Hydrogen peroxide reacts very fast. It disintegrate into oxygen and water, without the formation of byproducts. This increases the amount of oxygen in water. The disinfection mechanism of hydrogen peroxide is based on the release of free oxygen radicals.
The Legislation for Hydrogen Peroxide
In the USA, hydrogen peroxide is registered as a pesticide by the EPA in 1977. Hydrogen Peroxide is immediately dangerous to life or health at higher concentration (75 ppm)
Therapeutic Uses of H2O2
When it comes to hydrogen peroxide therapy there seems to be only two points of view. Supporters consider it one of the greatest healing miracles of all time.
When exposed to other compounds hydrogen peroxide dismutates readily. The extra oxygen atom is released leaving H20 (water). In nature oxygen (02) consists of two atoms a very stable combination. A single atom of oxygen, however, is very reactive and is referred to as a free radical. Over the past several years, we’ve continually read that these free radicals are responsible for all types of ailments and even premature aging. What many seem to forget, however, is that our bodies create and use free radicals to destroy harmful bacteria, viruses, and fungi. In fact, the cells responsible for fighting infection and foreign invaders in the body (your white blood cells) make hydrogen peroxide and use it to oxidize any offending culprits. The intense bubbling you see when hydrogen peroxide comes in contact with a bacteria-laden cut or wound is the oxygen being released and bacteria being destroyed. The ability of our cells to produce hydrogen peroxide is essential for life. H202 is not some undesirable by-product or toxin, but instead a basic requirement for good health.
Newer research indicates we need hydrogen peroxide for a multitude of other chemical reactions that take place throughout the body. For example, we now know that vitamin C helps fight infections by producing hydrogen peroxide, which in turn stimulates the production of prostaglandins.
There are references indicating use of hydrogen peroxide release during mouth rinsings on the composition of the microbiota of developing plaque in humans and the amount and pathogenecity of the plaque formed. The mouthwash which is used as the only oral hygiene significantly retarded gingivitis development.
Using Hydrogen Peroxide for Cleaning
Clear liquid, non volatile, non explosive, non inflammable and non toxic product that looks like water but with a great amount of oxygen, that´s why in many languages it´s name is “oxygenated water”, this product has a slight biting odor and a little bit irritating for the eyes, at the contact with the skin and the eyes it produces oxidation burns, so you must always wear rubber gloves. First off, it is anti-bacterial, anti-fungal, anti-mold and anti-mildew. Whew! so it makes sense to use it as a household cleaner. Using hydrogen peroxide for cleaning is practical, non-toxic and cheap! Hydrogen Peroxide can be used for cleaning tiles, stained toilets, stained plastic, carpet stains for using sodium percarbonate a powdered form of hydrogen peroxide. Just put some 3% hydrogen peroxide into a spray bottle. Spray it on and wipe it off as you would with other household cleaners. It can be bought in concentrated form and in bulk, using hydrogen peroxide for cleaning is very inexpensive. And, finally, using hydrogen peroxide for cleaning tends to keep your sponges, mops, and scrubie pads a whole lot cleaner. (They’ll all get a bit of disinfecting every time you use them.)
90% Hydrogen Peroxide: for Rocket Fuel
Believe it or not is used as an oxygen source for rocket fuel. The hydrogen peroxide rocket engines are, in fact steam rockets, but this steam is produced by a violent exothermic reaction of the peroxide. When passed through a catalyst pack, the peroxide decomposes into superheated steam and oxygen. This high pressure steam is expelled supersonically through a DeLaval nozzle, which produces thrust. For each volume of liquid injected at the catalyst, after the reaction you get 5000 times that volume in gas expelled at the nozzle. The Hydrogen Peroxide is the only product used in the reaction, this places it in the monopropellant liquid rocket fuel classification. This kind of rocket, whether using steam or hot water, is the safest of all the rocket engines. Sometimes considered a “cool rocket,” it does not produce flame, and therefore can be made of stainless steel. (Juan Manuel Lozano G. June 2008, Motociclismopanamericano)
Hydrogen Peroxide for Plants
Many farmers have been increasing crop yields by spraying them with diluted hydrogen peroxide (300 gms of 35% H202mixed with 500 lit of water per acre). You can achieve the same beneficial effect with your house plants by adding 30 gm of 3% hydrogen peroxide (or 16 drops of 35% solution) to a liter of water you give your plants.
(It can also be made into an excellent safe insecticide. Simply spray your plants with 225 gm of 3% peroxide mixed with 225 gm of white sugar and 5 lit of water.)
Slow releasing forms of Hydrogen peroxide wherever liquid hydrogen peroxide cannot be used, H2O2 is available in paste, honey, slow releasing solid forms.
Honey
Hydrogen peroxide is formed in a slow-release manner by the enzyme glucose oxidase present in honey. It becomes active only when honey is diluted, requires oxygen to be available for the reaction (thus it may not work under wound dressings, in wound cavities or in the gut), is active only when the acidity of honey is neutralised by body fluids, can be destroyed by the protein-digesting enzymes present in wound fluids, and is destroyed when honey is exposed to heat and light. Also, the antioxidant constituents in honey help clean up oxygen free radicals present.
C6H12O6 + H2O + O2 → C6H12O7 + H2O2 (glucose oxidase reaction)
When honey is used topically (as, for example, a wound dressing), hydrogen peroxide is produced by dilution of the honey with body fluids. As a result, hydrogen peroxide is released slowly and acts as an antiseptic.
Sodium Percarbonate
The name “sodium percarbonate” (SPC) does not reflect the structure of this oxidizing agent, which is in fact a carbonate perhydrate: 2 Na2CO3 • 3 H2O2. Although SPC is very storage-stable if dry, the solid material has a slight vapour pressure of hydrogen peroxide leading to exchange with water or to violent reactions with oxidizable substrates, even in the solid state.
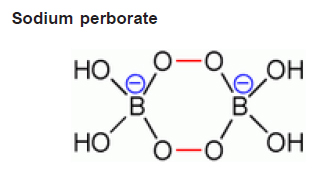
Unlike sodium percarbonate and perphosphate, the sodium perborate is not simply an adduct with hydrogen peroxide, but contains a cyclic anion (B2O4(OH)42-) with two peroxo bridges and does not contain the BO3" ion. This makes the substance more stable, and safer for handling and storage. The formulae of the mono and tetra hydrates can therefore be wr itten as Na 2 H 4 B 2 O8 (anhydrous) and Na2H4B2O8·6H2O respectively. Sodium perborate undergoes hydrolysis in contact with water, producing hydrogen peroxide and borate. It serves as a source of active oxygen in many detergents, laundr y detergents, cleaning products, and laundry bleaches. It is also present in some tooth bleaching formulas.
Manufacturers Information
The cost of 30 % Aq. H2O2 in bulk is available for approx. Rs. 40 per Kg (Ref. National Peroxide Ltd., Mumbai, India.)
Conclusion
Hydrogen Peroxide is non-toxic for people, plants, household animals, and the earth. Don’t forget that whatever we use do end up going back through the sewage system or otherwise to the earth, rivers, oceans and environment eventually. This chemical though a strong oxidizing agent has no harmful products except useful products like pure oxygen and water. Though one has to be careful in using this wonder chemical in appropriate dilutions.
The challenge is how to achieve the goals of conservation, sustainable development, and access and benefit sharing for biological resources and traditional knowledge. Thirteenth Finance Commission of India, states that in making its recommendations, the Commission shall have regard, among other considerations, to the need to manage ecology, environment and climate change consistent with sustainable development, and the need to improve the quality of public expenditure to obtain better outputs and outcomes.
References
- “Many benefits of H2O2” by – Dr. David G. Williams (2003).
- Hydrogen Peroxide - Curse or Cure ?
- “Aquaculture in India” – By Jitendra Nayak, 07 Jan 2006.
- “Technologies for a clean environment” – US Peroxides Inc.
- “Hydrogen Peroxide” – Wikipedia, the free encyclopedia
- “ Kirk – Othmer encyclopedia of chemical technology, 4th ed., Wiley, N.Y Vol 13 (1995).
- Houtmeyers, J. et al. “Hydrogen Peroxide as a supplemental Oxygen Source for Activated Sludge: Microbiological investigations”, European J. Appl. Microbiol. (1977).
- Cole, C. A. et al., J. Water Pollution. Contr. Fed. 46: 2579-2592 (1973).
- Http//www.using – Hydrogen Peroxide.com







