Formation of New Products in a Solid-Solid Reaction in the Presence of Organic Solvent Impurity
Rabia Ahmad1 * and Qamer Faisal1
1
Department of Chemistry,
Faculty of Natural Sciences,
Jamia Millia Islamia (Central University) Jamia Nagar,
New Delhi,
110 025
India
DOI: http://dx.doi.org/10.12944/CWE.6.1.16
We discuss the formation of new products in a solid – solid reaction in the capillary as in well as in bulk in the presence of an organic solvent impurity in a small amount. Three reactions namely KI + HgCl2, CuI + HgCl2 and AgI + HgCl2 were studied. It was found for some of the organic solvent impurities, a small amount of some substance appeared in the half occupied by HgCl2. Thus it became clear that some of the organic substances were reacting with HgCl2 to produce a new substance which be an organo-metallic product. In this light HgCl2 was made to react with the different solvents. However, the new product could be separated out only in two cases, with Cyclohexanone and Dimethylsulfoxide. A black coloured product was formed with Cyclohexanone and with Dimethylsulfoxide the new product was of a light greenish colour. With the other solvents like Acetophenone and Nitrobenzene, the product, if formed, could not be separated out. So a preliminary study of the two products naming these as product A and product B formed with Cyclohexanone and Dimethylsulfoxide respectively has been done. The product A is formed by the reaction of Cyclohexanone solvent with HgCl2 and product B is formed by the reaction of Dimethylsulfoxide solvent with HgCl2 both at 80ºC, for 5 hours. The preliminary studies carried out were measurement of molecular weight (by elevation of boiling point method), ionicity, XRD, FTIR etc.
Copy the following to cite this article:
Ahmad R, Faisal Q. Formation of New Products in a Solid-Solid Reaction in the Presence of Organic Solvent Impurity. Curr World Environ 2011:6(1);115-124 DOI:http://dx.doi.org/10.12944/CWE.6.1.16
Copy the following to cite this URL:
Ahmad R, Faisal Q. Formation of New Products in a Solid-Solid Reaction in the Presence of Organic Solvent Impurity. Curr World Environ 2011:6(1);115-124. Available from: http://www.cwejournal.org/?p=1295
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-04-12 |
|---|---|
| Accepted: | 2011-06-17 |
Introduction
Many solid-solid capillary reactions have been studied during the last many years e.g.1-4 In the studies normally carried out so for, both the solid reactants do not have any impurities. The main motivation of the earlier work, including that of the senior author (R.A.) in this field was to obtain a better understanding of the reaction mechanism of solid-solid reactions eg.2,5 Introduction of simple organic impurities was a step in this direction. While studying the solid-solid capillary reactions in the presence of small amounts of some organic solvent impurity, that a small amount of some substance appeared in the half occupied by one of the reactants. Thus it became clear that some of the organic substances were reacting with one of the reactants to produce a new substance which be an organo – metallic product.
Here, we discuss the formation of new products due to the presence of an organic solvent impurity in a small amount. The reactions studied are KI+HgCl2, CuI + HgCl2 and AgI + HgCl2 in the presence of small amount of organic solvent impurity.
The two reactants when packed side by side in a capillary tube of a narrow diameter, say 0.5 cm after moistening with different solvent impurities and kept in an oven maintained at 80oC showed some new product on HgCl2 side in two cases.
In the light of the indication from capillary studies HgCl2 was made to react with the different solvents. However, the product could be separated out only in two cases, with Cyclohexanone and Dimethylsulfoxide. A black coloured product was formed with cyclohexanone and with Dimethylsufloxide the new product was of a light greenish colour. With the other solvents like Acetophenone and Nitrobenzene, the product, if formed could not be separated out. So, we have made a preliminary study of the two new products, naming these as product A and product B respectively. The product A is formed by the reaction of Cyclohexanone solvent with HgCl2 and product B is formed by the reaction of Dimethylsufloxide solvent with HgCl2, both at 80ºC, for 5 hours.
Experimental
Method of Preparation
We take 4 gms of well ground HgCl2(99.9% Merck) reactants, moistened with about 0.4 ml of Cyclohexanone solvent (99% Merck) on a watch glass. Then it is put in an oven at constant temperature of 80º C, for 5 hours. In this way,a black coloured product is formed,this is called product A Same procedure is adopted for the Dimethylsulfoxide solvent (99% Merck). HgCl2 is moistened with 0.4 ml of the double distilled solvent and the same procedure is followed in this case also. A light greenish coloured product is formed, this is called product B. Powdered crystals of the new products are taken for observation, measurement and other studies with simple techniques for finding out their physical properties etc. These are given in Table 1.
Measurement of Molecular Weight (By Elevation of Boiling Point Method)
The equation relating the boiling point elevation with the molecular weight of the solute is given as
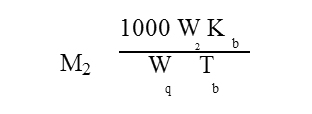
where M2 is the molecular weight of the solute, W2 is the mass of solute, Kb is the molal elevation constant, W1 is the mass of the solvent and Tb is the boiling point elevation due to the presence of the solute. THF is used as solvent.
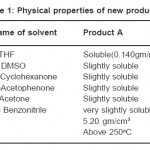 |
Table 1: Physical Properties of New Products Click here to View table |
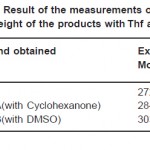 |
Table 2: Result of the Measurements of the Molecular Weight of the Products with Thf as Solvent Click here to View table |
Tb is measured by a digital electronic thermometer.
Benzoic acid and Acetanilide are taken as standard solute. A known small amount of the solute is added to known amount of THF solvent and the elevation of boiling point is found out. All chemicals used are 99% purity Merck chemicals.
The Kb occurring in the formula for the determination of molecular weight is found out using Benzoic acid and Acetanilide as solute. The mean value of Kb using Actanilide as solute is used for the determination of molecular weight. The mean value of Kb for THF is 191.45. These molecular weights are given in Table 2.
Measurement of Some Other Properties of this New Product Such as Ionicity, XRD, FTIR etc
The kinetics of the reaction is studied by placing 400 mgms of HgCl2and about the same amount of CuI in close contact near the middle, first without any impurity, and then after mixing with the two new products formed, as impurities, taken turn by turn. Both the reactants are 99.9% purity Merck chemicals. The internal diameter of the pyrex glass tube is 0.5 cm and about 5 cm length. These are kept in an air thermostat oven, controlled upto 0.5º C to 1ºC at 80ºC. The product thickness is measured with a traveling microscope having a least count of 0.01mm at equal intervals of time (15 min). The results are given in Fig. 1 and the corresponding digital data are given in Table 3.
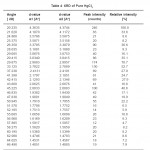 |
Table 4: XRD of Pure HgCl2 Click here to View table |
The X-ray diffractogram of HgCl2 and the two products are obtained. These are given in Figs. 2-4. The corresponding digital data are given in Tables 4-6. The digital data of some of the most prominent diffraction peaks is given in Table 7. The near – matching normal mode frequencies of HgCl2and the two products are given in Table 8 (a) and the remaining ones are given in Table 8 (b). The FTIR spectrum of HgCl2 and the two products are given in Figs. 5-7.
Discussion
We see from Table 7 that the prominent peaks of product A are very nearly at the same place as that of HgCl2. This means that HgCl2 molecule remains more or less as such in product A, with some organic group getting attached to the HgCl2 molecule, consistent with the molecular weight of product A. This proposal is consistent with observations because the organic group is expected to diffract X-rays very-very scantily.
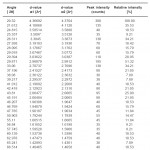 |
Table 5: XRD of product A Click here to View table |
On the other hand, for product B, the 100% intensity is diffracted for d=8.0289 which is a totally new d-value. Also there is a new peak of 34.8% intensity at d=3.4618. There is also a new peak of intensity 15% at d=2.7696. Rest of the prominent d values for product B are more or less same as for HgCl2 From this, we may conclude that at least one chlorine is replaced by an organic group consistent with the observed molecular weight. This should account for the observed X-ray data.
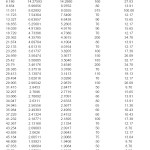 |
Table 6: XRD of product B Click here to View table |
From Table 8 (a), we also notice many near matching between the normal mode frequencies of HgCl2 and those of product A. There are fewer near matching between the frequencies of HgCl2 and those of product B. This is consistent with our proposal regarding the new products.
We may tentatively suggest the following structures for the new products. Then one should have a very close look at the FTIR and other supporting data to eliminate many of the proposed structures to hopefully arrive at the best choice.
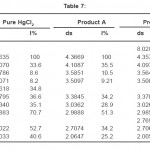 |
Table 7 Click here to View table |
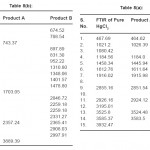 |
Table 8 (a) and (b) Click here to View table |
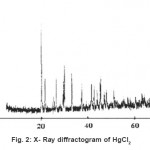 |
Figure 2: X- Ray diffractogram of HgCl2 Click here to View figure |
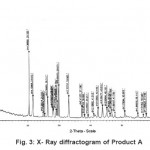 |
Figure 3: X- Ray diffractogram of Product A Click here to View figure |
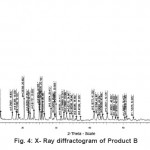 |
Figure 4: X- Ray diffractogram of Product B Click here to View figure |
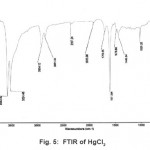 |
Figure 5: FTIR of HgCl2 Click here to View figure |
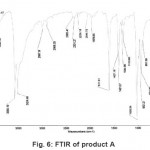 |
Figure 6: FTIR of product A Click here to View figure |
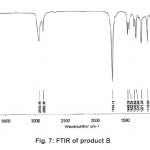 |
Figure 7: FTIR of product B Click here to View figure |
| Proposed structure of the new product being given heremerely as one of the examples | Formula weight | Observed Molecular |
| Product A (HgCl2 )CH3 SHO | 287.54 | 287.07 |
| Product B Hg SHO | 299.54 | 303.25 |
The above are merely examples and these are not even good examples. Out of the many proposals, that one may make, one should be able to arrive at the correct structure, mainly on the basis of the FTIR and other supporting data. That is not being done here.
The normal mode frequencies of the two solvents, Cyclohexanone and Dimethylsulfoxide can be seen from Fig. 8 and 9, where the frequencies are noted in digital form over the spectrum.
Concluding Remark
One structure for product A and one for product B are tentatively proposed. More such structures have to be proposed and assessed against the observed molecular weighty and the spectra etc. Only then one may arrive at a unique structure for each of the two products.
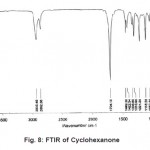 |
Figure 8: FTIR of Cyclohexanone Click here to View figure |
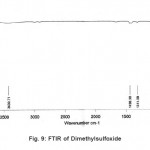 |
Figure 9: FTIR of Dimethy lsulfoxide Click here to View figure |
Acknowledgements
We wish to express our thanks to Prof. M.A. Wahab, HOD Department of Physics, JMI, New Delhi for X-ray measurement. Thanks are also due to Prof. Kamaluddin, Department of Chemistry, IIT Roorkee for X-ray and FTIR measurements. We would also like to thank Prof. Amir Azam for FTIR measurements. Special thanks are due to the Ex-Head of the Chemistry Department Prof. Kishwar Saleem, for extending to us the facilities of the Department. We are also thankful to Prof. Sharif Ahmad, Head Department of Chemistry, JMI, New Delhi.
Referenecs
- Beg M.A., Ansari S.M., J. Solid State Chem. 20: 103 (1977).
- Beg M.A., Saud R., Ind. J. Chem. 25A: 373 (1986).
- Beg M.A., Jain A., Polyhedron 11(21): 2775 (1992).
- Beg M.A., Ansari S.M., J. Solid State Chem. 18: 57 (1976).
- Rahman R., Ph.D. Thesis, Aligarh Muslim University, Aligarh (1984).
- Maron S.H. and Prutton C.F., Principles of Physical Chemistry, 4th ed, Amerind Publishing Co. New Delhi, Bombay, Calcutta, New York (1965).






