Physical and Chemical Properties and Algal Composition of Derbendikhan Lake, Sulaimania, Iraq
Janan Jabbar Toma1 *
1
Department of Environmental Sciences,
College of Sciences,
University of Salahaddin,
Hawler,
Kurdistan region of Iraq
Iraq
DOI: http://dx.doi.org/10.12944/CWE.6.1.02
Limnological studies of Derbendikhan Lake were carried out was carried out seasonally over period of 4 season (summer, autumn, winter and spring). The present study deals with physico - chemical parameters and algal composition. The maximum and minimum range of physico - chemical properties were as, 13-34 °C and 12-30 °C for air and water temperature, Electrical conductivity and total dissolved solids varied from 435-485µs/cm and 278-310mg/L respectively, pH on alkaline side of neutrality, alkalinity 200 - 240 mgCaCO3 / L in February and May respectively, harness ranged between 210-280mgCaCO3 /L, the highest value of calcium and magnesium reached to 70mg/L and 34mg/L respectively, minimum value of sodium was 7.0 mg /L and maximum value of potassium was 1.4 mg /L, sulfate value ranged from 125-175mg/L, minimum value of chloride was 24mg/L.Higer value of nitrite, phosphate and silicate was 0.86µgN-NO2 /L, 25µgP-PO4 /L and 0.5µgSi-SiO2 /L respectively.
A total of 30 species were recorded. Fourteen species of the total belong to Bacillariophyceae, 8 species belong to Chlorophyceae, 5 species to Cyanophyceae, 2 species to Pyrophyta, and 1 species to Euglenophyceae. K
Copy the following to cite this article:
Toma J.J. Physical and Chemical Properties and Algal Composition of Derbendikhan Lake, Sulaimania , Iraq. Curr World Environ 2011:6(1);17-27 DOI:http://dx.doi.org/10.12944/CWE.6.1.02
Copy the following to cite this URL:
Toma J.J. Physical and Chemical Properties and Algal Composition of Derbendikhan Lake, Sulaimania , Iraq . Curr World Environ 2011:6(1);17-27. Available from: http://www.cwejournal.org/?p=1327
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-03-27 |
|---|---|
| Accepted: | 2011-05-14 |
Introduction
Increasing population and higher levels of human activities including effluent disposals to the surface and groundwater resources have made sustainability of resources a very complex task throughout the world.1
Water is known to contain a large numbers of chemical elements .Physical parameters such as temperature, turbidity and current are also known to operate in Lake Ecosystem. The interaction of both the physical and chemical properties of water plays a significant role in the composition, distribution and abundance of aquatic ecosystem. Apart form this, it also gives an insight into the relationships between the organism and their environment and can be used in determining water quality, and productivity of the lake. The physico-chemical study could also help in understanding of the structure and function of a particular water body in relation its habitants.2 The proper balance of physical, chemical and biological properties of water in lake is an essential ingredient for successful production of aquatic resources.2
Water is one of the important sources, to sustain life and has long been suspected of being the source of much human illness. Source of surface water and ground water have become increasingly contaminated due to increased industrial and agricultural activity. The public desires water that is low in hardness and total solids, non-corrosive and non-scale-forming.
A fascinating thing about lakes is that they provide their own variety. The task of limnologist is to measure and interpret this variation whether it concerns physical, chemical, biological phenomena, altitude, geology of the catchments area and the depth of water.3
Increased use of lakes and reservoirs for recreation, fisheries, water storage for irrigation and electric power generation purposes as well as for urban shoreline development, has emphasized the need for intensive water quality studies and management.4 The physical and chemical limnology of a lake is characterized by hydrologic impact, autogenic nutrient dynamic and biological aspects. These factors combine with each other determine the water quality and consequently community of the lake.5 Iraqi Kurdistan is considered a rich area with water resources if compared to its size, the water resource is available in different forms, including surface water (river, stream, spring, Kareeze and lake), groundwater, rain and snow, the second important lake is Derbendikhan reservoir which was constructed on Serwan river, this reservoir was established for flood control, irrigation, power generation, fish wealth, in addition to improving environmental and developing tourism, through the last 10 years or more, the water level is reduced in all lakes in the region, particularly in Derbendikhan lake, the reason is back to the low precipitation rate, which lead to increasing the levels of pollutants, thus the object of this study is to determine the physico-chemical properties and algal composition and assessment the water quality of the lake.
Description of the Area
Derbendikhan Lake (Fig. 1) is located on the southeast of Sulaimani, boundaries extended from (latitude 35° 6¹- 35° N and longitudinal 45°41¹-20° E), and the second largest lake in the Iraqi Kurdistan region at an altitude of about 485m above sea level.6 The area of this water lake is about 114.30K2 with the maximum depth of 75m.7 The estimated volume of the Derbendikhan lake ranges from 1.3-1.4Km3.6 Consequently Derbendikhan can be classified as limnetic water body, as warm and monomictic with only one circulation period in winter and water temperature never falls below 4°C.8 Geology of the area consists of sedimentary rocks of marine origin.6 The collection decision of9 regarding the climate of the studied area is a dry-summer approaches Irano-Turanian type characterized by the occurrences of three seasons: a cold winter, mild growing period of spring and hot-dry summer. Two sites in Derbendikhan mid lake and lake outlet were carried out seasonally variation over period of 4 seasons (15-8-2008, 15-11-2008, 15-2-2009 and 15-5-2009) respectively (Fig. 1).
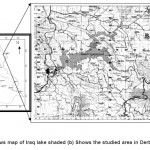 |
Figure 1: (a) Shows map of Iraq lake shaded (b) Shows the studied area in Derbendikhan lake Click here to View figure |
Material and Methods
Seasonally surface water samples were taken from two selected site in Derbendikhan lake(one in mid lake and the second in the lake outlet main channel) seasonally over period of 4 seasons (15-8-2008, 15-11-2008, 15-2-2009 and 15-5-2009) respectively. Air and water temperature was measured immediately in the field by placing a clean mercury thermometer (0-60 °C) graduated up to 0.1 °C inside the water. EC, TDS & pH were measured by using (pH-EC-TDS meter, HI 9812, Hanna instrument). Alkalinity, total hardness, dissolved oxygen , Ca+2 ,Mg+2 ,Na+1, K+1,sulfate and chloride were estimated.10 Nitrite, phosphate and silicate were estimated.15 Identification of algae was carried out using compound microscope model, Olympus in accordance to the available reference identification text books.11-14
Results and Discussion
Lakes are an important natural resource for population lived around the vicinity. They provide water for domestic and industrial uses, fisheries and irrigation. Knowledge of Lake Hydrology is essential for their proper use and conservation. The physicochemical parameters and nutrient content of water play a significant role in the distributional patterns and species composition of primary organisms Sahato et al.,.15 In aquatic habitats, environmental factors include various physical properties of water, such as solubility of gases and solids, light penetration, temperature and density. Chemical factors such as pH, hardness, phosphate and nitrate are very important for growth and dispersal of the phytoplankton depend for their existence. The results (Tables 1, 2, 3 & 4) showed that the parameters studied were effective in identifying and monitoring water bodies affected by organic sewage discharges. Hydrographic condition of Derbendikhan Lake, seasonal and physico-chemical change in water surface affect the abundance and distribution of biodiversity.
Tables (5, 6, 7 & 8) show the significant differences between the two sites for different physico-chemical characteristics, that sows found significant differences between the sites in some characteristics and not found in others characteristics, and these clear phenomena in these tables.
Temperature is important in terms of its effect on aquatic life. Temperature fluctuations are evident seasonal patterns in aquatic ecosystems. Its influence on limnological phenomena such as stratification, gas solubility, pH, conductivity and planktonic distribution are well known Singh et al.,.16 Temperature measurements are useful in indicating trends for various chemical, biochemical and biological activities. An increase in temperature leads to faster chemical and biochemical reactions. The growth and death of microorganisms and the kinetics of the biochemical oxygen demand are also regulated to some extent by water temperature Khuhawar and Mastoi.16 The air temperature showed an annual variation about (13–34 °C) at the sampling sites in Derbendikhan Lake. A higher air temperature was observed in August, particularly at site one during 2008, while the minimum value recorded in February, particularly in site one during 2009. In this study fluctuation of air temperature depend on the climate of the areas which closely approaches the Irano-Turanian type. The climate of the area characterized by semi-arid type with a wide seasonal range in air temperature. Statments by (9 and 17), seem to confirm the present conclusion.
Water temperature in the study lake was closely followed by air temperature, with maximum in summer and minimum in winter. Air and water temperatures showed a very characteristic annual cycle, with higher values during the winter was 30°C and lower values in the summer was 12°C. This variation may be related to many environmental factors such as elevation, current velocity, water depth; bottom materials expose to direct sun light and degree of shading or vegetation cover, evaporation and wind speed (18 and 19).Similar conclusion made by (20 and 4).
The results indicated that the water of Derbendikhan lake was rich and high dissolved solids
Surface water samples of Derbendikhan lake appeared an electrical conductivity of 400-485µs/cm. The highest value recorded in October-2008, this may be related to evaporation, however the period of this study characterized by low precipitation and relatively was hot, while the lowest value recorded in February-2009, possibly related to high precipitation and cause dilution to lake water.21 Similar conclusion made by (4 and 22).
The highest value of EC recorded in site two, this probably related to that profundal zone of Derbendikhan lake which contained high amounts of salts and dissolved materials setting in the lake bottom.21
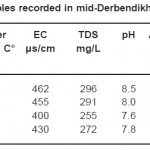 |
Table 1: Physico-chemical variables recorded in mid-Derbendikhan Lake during the study period Click here to View table |
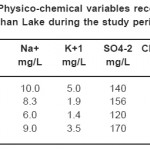 |
Table 2: Physico-chemical variables recorded in mid-Derbendikhan Lake during the study period (continue) Click here to View table |
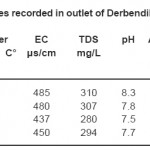 |
Table 3: Physico-chemical variables recorded in outlet of Derbendikhan Lake during the study period Click here to View table |
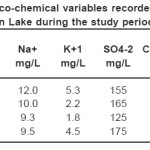 |
Table 4: Physico-chemical variables recorded in outlet of Derbendikhan Lake during the study period (continue) Click here to View table |
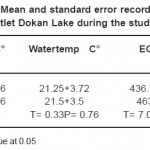 |
Table 5: Mean and standard error recorded in mid and outlet Dokan Lake during the study period Click here to View table |
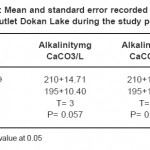 |
Table 6: Mean and standard error recorded in mid and outlet Dokan Lake during the study period Click here to View table |
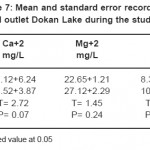 |
Table 7: Mean and standard error recorded in mid and outlet Dokan Lake during the study period Click here to View table |
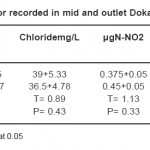 |
Table 8: Mean and standard error recorded in mid and outlet Dokan Lake during the study period Click here to View table |
The minimum amount of total dissolved solids (TDS) was recorded in winter (272mg/L) during2008and the maximum amount in summer and March (310mg/L), during 2009.This variations may be due to the size of the water body, inflow of water, consumption of salt by algae and other aquatic plants, and the rate of evaporation.16 A rapid increase in TDS occurs in the hot season due to an increase in the amount of bicarbonate. However, during the rainy season the amount of TDS was low due to dilution effects.16
pH is a measure of the acidity or alkalinity of an aqueous solution. Most aquatic organisms can tolerate to reasonable pH range (6.0-9.0), but they are more active when the pH value is around 7.23 Iraqi inland waters including that of Kurdistan have an alkaline status, because of its geological formation, and soil and minerals properties of mountainous areas.24 Surface water samples of Derbendikhan lake showed pH value at the range of 7.5-8.5. The difference between pH values at two sites in various seasons of the year was significant. This variation in pH is due to the presence or absence of free carbon dioxide and carbonate and planktonic density during various months.16 The highest pH value was recorded during summer, this might be attributed high photosynthetic due to the abundance of the algal population and increase of carbonate.21 The low pH values in winter could be related to the overturn period and increase of rainfall both of which lead to reduction in pH value.25 Similar conclusion made by (4, 20 and 22). The lowest pH value was always recorded in site two , this probably related to that profundal zone of Derbendikhan lake actually contain low phytoplankton density and high organic matter, which increase of CO2, therefore may caused a decreased of pH value.26
The capacity of water to neutralize a strong acid is known as alkalinity and is characterized by the presence of hydrogen ions; most of the alkalinity of water is due to dissolution of carbonate.16 In this survey the maximum alkalinity value 250 mgCaCO3 /L was noted at site 1 in October-2008 and minimum value of alkalinity was noted 170 mgCaCO3 /L was noted at site 2 in February-2009. Increase in alkalinity values may be due to decreases in the water level. Bicarbonate increases with decreases in water levels have also been reported.16 That the accumulation of lesser quantities of carbonate during summer results in the liberation of free CO3 during decomposition of bottom deposits, which possibly converts insoluble CaCO3 into soluble Ca (HCO3)2. Increase in alkalinity values may be related to phytoplanktonic activity such as photosynthesis and respiration processes.Whilest the lower value calculated in February-2009, this may be attributed to the dilution phenomena caused reduction in alkalinity.
The increase in the bicarbonate during autumn may be attributed to the decrease in air and water temperatures, leading to a decrease in the reaction rate of carbonate direction and vice versa. On the other hand, the increase in the HCO3 ¯ values were recorded during spring may be attributed to the flourishing of phytoplankton and the increase in the photosynthetic process leading to liberation of CO2 which is converted into bicarbonate. Also the lower values of HCO3 ¯recorded during winter, and not detected during autumn, this is mainly attributed to the decomposition in the dead phytoplankton leading to liberation of CO2 which dissolves in water and increase in the formation of HCO3¯.23 Generally, the high bicarbonate values of the three ponds indicate their high productivity and consequently favorable contribution for fish production23. These results agree with that finding by27 on Lake Manzalah. Similar conclusion investigated by (4 and 22). The lowest value of alkalinity recorded in site two, this probably related to that bottom zone of Derbendikhan lake content was low oxygen and high in organic matter.26
A lake’s hardness is affected by the type of minerals in the soil and watershed bedrock, and by the amount of lake water coming into contact with these minerals. If a lake gets groundwater from aquifers containing limestone minerals such as calcite (CaCO3) and dolomite (CaMgCO3), hardness and alkalinity shall be high.28 Total hardness content in Lake Derbendikhan reached its minimum value (220mgCaCO3/L) during February-2009 and its maximum (290 mgCaCO3/L) in September-2008.The highest value probably related to evaporation which increase and concentrate the available cations, while the lower value may be related to the rainfall and dilution of cations content.23
The results of dissolved oxygen show slight seasonal and regional variations in ranging from (4.5-9.5mg/L) in summer and winter respectively. The distribution of DO is affected by the solubility of many inorganic nutrients, which are governed by seasonal shifts from aerobic to anaerobic environments in some regions of the three ponds.29
The lowest dissolved oxygen values were recorded in the lake. In summer dissolved oxygen showed lower values than the other seasons, this may be due to several factors, the rise in temperature, increased biological activity, respiration of organisms and the increased rate of decomposition of organic matter.23
The distribution of calcium and magnesium concentrations in the water of Derbendikhan Lake was highly fluctuations during different periods. However, the calcium values were found higher than magnesium during the studied period.
Calcium is essential for all organisms, being an important cell wall constituent and regulates various physiological functions in animal too. The mean values of calcium ranged between 42.1-72.1 mg/l. The calcium content was found to increase during the summer and decrease during the winter and spring. This fall in Ca++ concentration during winter period may be attributed to dilution factor and comparatively high temperature during summer which is known to increase Ca++ concentration in water.23
The lower values of the calcium were recorded during spring may be attributed to the uptake of the calcium by microorganisms.30 However, the high calcium contents were recorded during summer and autumn may be related to the relative increase in the temperature then increase of evaporation.31 Generally, the calcium contents in the water is affected by the adsorption of the calcium ion on the metallic oxides32 in addition to, the effect of the microorganisms which play an important role in the calcium exchange between sediment and overlying water33. The variation in magnesium concentration in this studied area may be related to this may be attributed to, MgCO3 is partially soluble during these seasons and the magnesium precipitated as Mg (OH)3.34 On the other hand, the slight variations in the distribution patterns of the magnesium during summer and autumn. This is mainly attributed to its minor biotic demand and high solubility characteristic of they salts that keeps a homogenous distribution and mass balance for magnesium contents during these seasons.35
The present results show a slight variation in the sodium distribution patterns during winter, spring and summer. This is mainly attributed to the high solubility of them salts that keep a homogenous distribution and mass balance for sodium.35 However, the high values of the sodium contents ranged from 6-12 mg/l during autumn may be due to the release and the dissolution of the sodium ions from sediment and rocks into the overlying water.
The slight seasonal variations in the potassium of the ponds, indicate that the conservative nature of K. The ranges of K+ were found to be from 1.4 – 5.3mg/L, the highest values recorded in summer and lower value estimated in winter may is due to the to evaporation which increase and concentrate the of potassium, while the lower value may be related to the rainfall and dilution of cations content.23 Sodium and potassium concentrations of the water lake followed the same seasonal trend.
The sulfate and chloride values were fluctuated in the ranges from (120-165mg/L) and (26-50mg/L) respectively in this survey. The seasonal variations of sulphate concentrations present in Tables (1-4) showing that, the distribution of sulphate during winter, spring and summer are similar to each other. However, the highest values were recorded during autumn. The relative increase in the chloride and sulphate concentrations during hot period may be due to the increase in the air and water temperatures followed by the high evaporation rate. These results agree with that reported by.36 On the other side, the high values in the sulphate and chloride concentrations unexpected during autumn. This is mainly attributed to the dissolution of some ions especially Cl-, SO4
— from the surrounding rocks and sediment which release into the water of the three ponds.
Nutrient salts (NO2-, NO3-, NH3-, PO4-, T.P and SiO2-) are plays an important roles in the productivity of the aquatic ecosystems supporting the food chain for phyto and zooplanktons as well as fish.37
The seasonal variations of nitrite shows that there is a relative increase in the NO2- contents during winter and very high during autumn, (Tables 1, 4). This is mainly attributed to the oxidation of existing ammonia, yielding nitrite as intermediate state especially in abundant oxygen during winter.35 This explanation is documented by the decrease in the ammonia concentrations during the same period. On the other side, there is a relative decrease in the nitrite contents during hot period, (Tables 1 - 4). This is probably due to they reduction into ammonia (this is supported by the relative increase in the ammonia concentrations) during this period. As well as, they uptake by the phytoplankton in the surface water.
The cycling of phosphorus within lakes and river is dynamic and complex, involving adsorption and precipitation reactions, interchange with sediments and uptake by aquatic biota.38
The seasonal variations of PO4 - concentration were found to be from 14.5-25 µgP-PO4/L during the studied area. The decrease in the PO4-values during summer season were probably due to the distinct drop in phytoplankton biomass, on which nutrient regeneration process depends. Also, the recycling processes of the nutrients depended on the nutritional status of algal cells.39 In addition to, the weak mixing in the water and sedimentation of the phosphorus at high pH of the ponds (during summer) by combination with iron as ferric phosphate.40 On the other side, the relative increase in PO4-during winter, can be related to the complete mixing of the water column and more phosphorus release from the sediment, especially in presence of dissolved oxygen as reported by.36
The seasonal variations of reactive silicate in the water of Derbendikhan Lake were found to be in the ranges from 0.22-0.55 µgSi-SiO2/L during this survey. The obvious increase in reactive silicate during hot period, especially summer, may be due to the increase in the dissolution of the diatoms frustules at high temperatures.41 Consumption and fragmentation of the diatoms frustules can accelerate the dissolution process of silicate by zooplankton.42 In addition to, the alkaline pH of the water accelerates the release of the silicate from sediments to the overlying water.35 The pronounced decrease in the reactive silicate of the water ponds during winter may be attributed to the uptake by the diatoms blooms, fungi, algae and phytoplankton and zooplanktons as well as fish.
Phytoplanktons were collected from the study sites of Derbendikhan Lake during the period of study. A total of 31 species were recorded of which 17 species belong to Bacillariophyceae (4species belong to centrals and 13 to Pennales), 7 species belong to Chlorophyceae, 6 species to Cyanophyceae, and 1 species to Euglenophyceae.
According to the present results, it seems that Derbendikhan lake and its outlet were generally dominanated by diatoms, similar conclusion made by (4 and 22).
As stated by (25 and 18), diatoms dominated numerically in the studied area. The dominancy of diatom species might be connected to their tolerance range to different environmental conditions as well as to availability of silica in lake.19
The highest peak in phytoplankton was recorded during October-2008, while the second peak was during March-2009. Statement that of (19) seem to confirm the present result, however as he stated that diatoms may dominant during winter and continue to be a major component of the flora in spring and early summer, although the species composition changes.
From phytoplankton composition encountered in this work, it seems that among diatom species Cyclotella ocellata and Melosira granulata were see the most common taxa in this survey. Similar conclusion made by (4 and 22).
On the other hand, nondiatom taxa in this study were seemed to be dominanated by Chlorophyta in particular in hot months such as Scenedesmus quadricauda and Scenedesmus bijuga are known to be planktonic in tropic lakes and oligotrophic lakes which are characterizes with poor status of nutrient.
Cyanophyceae as stated by (11) usually occur in abundance only during the warm month’s o a year, this was the situation in Derbendikhan Lake.
Euglenophyceae in this survey observed in autumn and spring, this can be connected to organic pollution.18
Conclusion
- The climate of Derbendikhan lake dose approaches to Irano-Turanian type;
- With regard to nutrient enrichment status Derbendikhan Lake can be Mesotrophic Lake.
- Calcium was the most dominant cations followed by magnesium the sodium and potassium.
- Bacillariophyceae considered the most abundant class in this study.
List 1
The non-diatom species recorded in mid
and outlet of Dokan Lake during the studied period
Division : CYANOPHYTA
Class: Cyanophyceae
1-Order: Chroococales
1- Family: Chroococaceae
1-Chroococcus minor (Ktz.) Naegeli
2-Meismopedia elegans A. Braun
2-Order: Oscillatoriales
1- Family: Oscillatoriaceaea
3-Lyngbya sp
4-Oscillatoria amoena (Ktz.) Gomont
5- O. limnetica Lemmermann
6- O. splendida Greville
Division: CHLOROPHYTA
Class: Chlorophyceae
1-Order: Chlorococcales (Chlorosphaeroles:
Chlorosphaeraceae)
1- Family: Oocystaceae
1-Chlorella vulgaris Bejerinck
2-Scenedesmus bijuca (Trup)
3- S. quadricauda (Turp.) de Brebisson
2- Order: Siphonocladales (Cladrophorales)
1-Family: Cladophoraceae
4-Cladophora glomerata (L.) Ktz
2-Order: Ulotrichales
1-Family: Ulotrihaceae
5-Ulothrix sp
12- Order: Zygnematales
1-Family: Desmdiaceae
6-Cosmarium sp
7-Spirogyra sp
Division: EUGLENOPHYTA
Class: Euglenophyceae
1. Order: Euglenales
1- Family: Euglenaceae
1-Euglena gracilis Klebs
List 2
The diatom species recorded in mid and outlet of Dokan Lake during the studied period
Division CHARTSOPHYTA
Class: C. Bacillariophyceae(Diatomaceae)
Order: 1. centrales
1-Cyclotella ocellata Pantocsek
2-Melosira granulata (Ehr.) Ralfs
3-M. varains Agardh
4-Stephanodiscus astrea (Ehr.) Grun
Order2: Pennales
5-Amphore ovalis (Ktz.) Kuetzing
6-Cocconeis placental Ehrenberg
7-Cymbella affinis (Kuezing)
8-C. tumida (Breb.) van. Heurck
9-Diatoma hiemale (Roth) Heiberg
10- D. vulgare Bory
11-Fragilaria capucina Desmazieres
12-Gyrosigma acuminatum (Ktz.) Rabenhorst
13-Navicula sp
14-Nitzschia sp
15-Surirella ovalis Brebisson
16-Syndra ulna (Nitz)
17-S ulna Ehrenberg
References
- Hammer, M.J and Warren, V.J., Water Supply and Pollution Control.7th Edition. Pearson Prentice Hall. Upper Saddle River. 867pp (2005).
- Hutchison, G.E., A treatise on Limnology Vol.1.Geography, Physics and Chemistry. John Wiley and Sons Inc. New York. 1011pp (1957).
- Stewart, K.M and Hasler, D. (1972).Limnology of some Madison lakes: Annual cycles. Wis. Acad.Sci.Arts Lett.60:87-123.
- Toma, J.J. Limnological study of Dokan Lake, Kurdistan region of Iraq. M.Sc. Thesis.Unversity of Salahaddin.Arbil. Iraq (2000).
- Welcomme, R.L., Ryder, R.A and Sedell, J.A. Dynamics of fish assemblages in river systems.Can. Spec. Publ. Fish. Aquat. Sci. 106: 569-577 (1989).
- Anon. The World Bank, Dokan and Derbendikhan Emergency Hydropower. Consultancy Services for Dokan and Derbendikhan Dam Inspections, Inspection report (final), E1537.SMEC.Section A-Complementary Grouting in Dam Main Remedial Works (Phase1), Analysis of Dam Behavior, Review of Previous Reports Recommendations, Coyne at Bellier. May. 1975 (2006).
- Al-Sahaf, M., Water Resources in Iraq. Ministry of Information. Baghdad. Iraq. 230pp (1976).
- Szczerbowski, J.A.; Bartel, R.; Ciepielewski, W., Hydrological characteristics of Dokan and Derbendikhan Dam and lakes of Tharthar, Habbaniya and Razzazah.Polish Fisheries. 9(1): 7-18 (2001).
- Guest, E., Flora of Iraq.Vol 1 .Ministry of Agriculture. Baghdad. 213pp (1966).
- A.P.H.A.American Public Health Association., Standard Methods For The Examination of Water and Wastewater .20th Edition. A.P.H.A. American Water Works Association. (A.W.W.A), and Water Pollution Control Federation, Washington, D.C (1998).
- Smith, G.M., The Fresh Water Algae of United State .Mc Graw-Hill. 719pp (1950).
- Desikachary, T.V., Cyanophyta. Indian Council of Agriculture Research. 686pp (1959).
- Prescott, G.W., Algae of the Western Great Lake Area.William.Brown Co. Publishers. Dubugue.Lowa. 977pp (1973)..
- Patric, R. and Reimer, C.W., The Diatoms of the United State. 1. Monger. Acad .Nat. Sci. NewYork .680pp (1966).
- G. A. Sahato, K. H. Lashari and S. B. Sahato., Hamdard Medicus vol. XLVII No (4) 100 (2004).
- Lashari, K. H.; Korai A. L.; Sahato G. A. and Kazi T. G., Limnological Studies of Keenjhar Lake, District, Thatta, Sindh, Pakistan. Pak. J. Anal. Environ. Chem. 10(1-2): 39-47 (2009).
- Al-Shalash, A.H., The climate of Iraq. The Cooperative printing, Presses Workers Society.Amman.Jordan.85pp (1966).
- Hutchinson, G, E., A treatise on Limnology. vol 2.Wiley. 1115pp (1967).
- Bartram and Balance, R., Water Quality Monitoring (a practical guide to the design and implementation of freshwater quality studies and monitoring).E and FN Spon, an imprint of Chapman and Hall. London. U.K.383pp (1996).
- Rasheed, R.O, Evaluation of heavy metals and polyaromatic hydrocarbons in water, fish, and sediments within Derbendikhan reservoir. Ph.D.Thesis.University of Sulaimania. Iraq (2008).
- Reid, G.K., Ecology of Inland Water and Estuaries. Reinhold Publishing Corporation. NewYork.375pp (1961).
- Shaban, A.A.G., An ecological study on phytoplankton in Dokan Lake. M.Sc. Thesis.Univ of Salahaddin. Arbil. Iraq (1980).
- Goldman, C. R. and Horne, A. J., Limnology. International student Edition, McGraw-Hill International Book Company, London, 197-220 (1983).
- Al-Saadi, H.A.; Al-Daham, N.K., and Al-Hassan, L.A., Aquatic Ecology.Ministryof H.E., and Sci.Res.Basrah Univ (1986).
- Welch, P.S., Limnology, 2nd Edition New York, Toronto, and London, Mc Graw- Hill, Book Company, Inc., 538 p (1952).
- Golterman, H.L., Chemistry.Chapt.2.In River Ecology.Ed..By B.A.Witton:39-80.Studies in Ecology.Vol.2.Wat.Res. 10:97-104 (1975).
- Abdel-Satar, A. M., Environmental studies on the impact of the drains effluent upon the southern sector of Lake Manzalah. Egypt. J. Aquat. Biol. & fish, 5(3): 17-30 (2001).
- Yalçýn Tepe1., Aysun Turkmen., Ekrem Mutlu1and Alpaslan Ate., Some physicochemical characteristics of Yarseli Lake, Hatay, Turkey. Turkish Journal of Fisheries and Aquatic Sciences 5: 35-42 (2005).
- Benson, B. B. and Krause, D. J., The concentration and isotopic fractionation of gases dissolved in freshwater in equilibrium with the atmosphere. Oxygen. Limnol. Oceanogr, 25: 662-671 (1980).
- Bowling, D. J. F., “Uptake of ions by plant roots” Champion and Hall. London (1975).
- Cole, G.A., Text book of Limnology. 2nd Edition, St. Louis, Toronto, London (1979).
- Wilson, T. R. S., “Salinity and the major elements of seawater” chemical oceanography. London, 1: 365-413 (1975).
- Elewa, A. A., Influence of oxidation reduction potential in the water environmental of Aswan High Dam Reservoir Water Res. Development., 4(3): 184-190 (1988).
- Goher, M. E. M., Chemical studies on the precipitation and dissolution of some chemical elements in Lake Qarun, Ph.D. Thesis. Fac., of Sci., Al-Azhar Univ., Egypt (2002).
- Wetzel, R. G., Limnology, saunder college publishing. 2nd ed. 767 pp (1983).
- Abdel-Satar, A. M., On the water quality of Lake Bardawil, Egypt. J. Egypt Acad. Soc. Environ. Develop, (Environmental studies), b(1): 49-73 (2005)
- Abdo, M. H., Environmental studies on the River Nile at Damietta branch region, Egypt. J. Egypt. Acad. Soc. Environ. Develop. (D-Environmental Studies), 5(2): 85-104 (2004).
- Broberg, O. and Persson, G., Particulate and dissolved phosphorus forms in freshwater: Composition and analysis. Hydrobiology, 170: 61-90 (1988)
- Lehman, J. T., Release and cycling of the nutrients between planktonic algae and herbivores, Limnol. Oceanogr. 25: 620- 632 (1980).
- Mesnage, V. and Picot, B, The distribution of phosphate in sediments and its relation with eutrophication of a Mediterranean Coastal Lagoon, Hydrobiology, 297: 29-41 (1995).
- Shabana, E. E. (1999): Limnological studies on Lake Bardawil, M. Sc. Thesis, Fac. Sci., Suez Canal Univ., Egypt.
- Ferrent, J. G. and Barker, J., The influence of planktonic and benthic crustaceans on silicon cycling in Lake Michigan, U.S.A., Verh. Int. Limnol., 20: 324 (1978).







