Physico-chemical Assessment of Ground Water in Dhule city of Maharashtra, India
R. C. Chautmal1 * , N. J. Biraris2 and T. J. Patil3
1
Department of Chemistry,
GET’s Art’s Commerce and Science College,
Nagaon,
India
2
Department of Applied Sciences,
SSVP’ BSD College of Engineering,
Dhule,
424002
India
3
Department of Chemistry,
JET’s Zulal Bhilajirao Patil College,
Dhule,
424002
India
DOI: http://dx.doi.org/10.12944/CWE.6.1.29
Ground water samples of the selected locations in Dhule city were analyzed in respect of physico-chemical aspects in order to determine the potability of the water and call for same effective measures to be taken to minimize the adverse impact on human health. The avarage values of assessment were compared with the standards given by WHO, ISI and ICMR. It was found from present study that there are variations in many physico-chemical parameters. The sample S-1 and S3 were found within the possible limits, while S-2 and S-4 shows large variations and found to be exceeding their acceptable limits of standards indicating poor potability due to contamination from sewage.
Copy the following to cite this article:
Chautmal R.C, Biraris N.J, Patil T.J. Physico-chemical Assessment of Ground Water in Dhule city of Maharashtra, India. Curr World Environ 2011:6(1);187-190 DOI:http://dx.doi.org/10.12944/CWE.6.1.29
Copy the following to cite this URL:
Chautmal R.C, Biraris N.J, Patil T.J. Physico-chemical Assessment of Ground Water in Dhule city of Maharashtra, India. Curr World Environ 2011:6(1);187-190. Available from: http://www.cwejournal.org/?p=1321
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-04-20 |
|---|---|
| Accepted: | 2011-05-29 |
Introduction
Water is elixir for the life. Adequate supply of potable water is absolutely essential and is the basic need for all human being on the earth. Attention on water contamination and its management has become a need of the hour due to its far reaching impact on human health.1,2 Ground water quality of open wells as well as bore wells deteriorates to a considerable extent and the water from such sources has high salt contaminations.
Ground water acts as reservoir by virtue of large pore space in the earth material, as it percolates over large distance and as mechanical filter which improves water quality by removing suspended solids and bacterial contamination. However with increase in demand due to population pressure this source is now a day over exploited in many parts on the earth.3 Urban sewage, improper management of municipal solid waste is the leading cause of the contamination in the ground water.
The main objective of the present study is the evaluation of physico-chemical aspects of ground water from some selected locations in Dhule city, to specify accurately and timely information regarding the potability of ground water. The present findings may be helpful to shape sound public policy and to implement water quality improvement program effectively as well as efficiently.
Study Area
Dhule city is divided into two main areas viz. Deopur and Dhule city separated by a river PANZARA. Both these areas are residential zones of major population. Many House-hold industrial effluents and urban sewage were continuously added through drainage into the river without any proper treatment. People residing in these areas utilize ground water for their daily needs. Sampling locations were selected on the basis of the detailed survey of the study area and discussions with local experts from water department of Municipal Corporation. The details’ regarding the locations is given in Table 1.
Table 1: Details of Sampling Locations
| Sample No. | Name of Location |
| S-1 | Forest Colony |
| S-2 | Borse Nagar |
| S-3 | Laxmi Nagar |
| S-4 | Varkhede Shivar |
The duration of sampling is during the months of October to December. All the samples were collected in clean polythene cans of one liter capacity and brought to the laboratory without the addition of any preservatives and subject to the physico-chemical analysis within 24 hours after collection. Suspended matters if any, in these samples were removed by filtration through the Whatman filter paper No. 41. All chemicals used were A.R.grade; Double distilled water was used for the preparation of various solutions.
Experimental
The samples collected were subjected to various physico-chemical analyses in order to assess their quality and potability. Water samples were analyzed using standard methods4-6
Physico-chemical parameters such as Temperature, pH, Electrical Conductivity, Total Dissolved Solids, Dissolved Oxygen, Total Hardness, Total Alkalinity, Calcium, Magnesium, Sodium, Potassium, Chloride, Nitrate, and Sulphate were determined using standard methods.5
Results and Discussion
The values obtained for various Physico-chemical parameters after analytical determination are given in Table 3. The values were compared with the standard values given by WHO7, ISI8 and ICMR9 as given in Table 2.
Table 2: WHO, ISI and ICMR Standards for Potability
| Parameter | ISI Maximum Permissible Limits | ICMR Maximum Permissible Limits | WHO Guidelines |
| PH | 6.5 – 8.5 | 6.5 – 9.2 | 7.0 – 8.5 |
| EC | 250 | 250 | 300 |
| TDS | 500 | 1500-3000 | 500 |
| DO | 5 | 5 | 5 |
| TH | 300 | 600 | 600 |
| Na+ | - | - | 250 |
| K+ | 45 | - | 45 |
| Ca++ | 75 | 200 | 200 |
| Mg++ | 30 | 50 | 30 |
| Cl- | 250 | 1000 | 250 |
| SO 2-4 | 150 | 400 | 400 |
(All Values in mg/L except pH and EC)
All the four samples were not having any objectionable colour appearance, taste or odour. The PH is measure of acidity or alkalinity. The PH values of water samples analyzed vary from 6.8 to 7.9 and were found to be within the permissible limits.
Electrical conductivity (EC) is an index to represent the total concentration of dissolved salts in water. This is confirmed by the fact that these samples were found to have fairly high values of TDS also. It is observed that water with high values is predominant in sodium and chloride ions10. When the concentration of both the ions was considered together, all the samples with high EC were found to have both the ions at higher concentration. The EC variation in the sample analyzed is shown in Fig. 1.
Table 3: The Average Values of Physico-Chemical Parameters in the Study Area
| Sample | PH | EC | TH | D | TDS | Na+ | K+ | Ca2+ | Mg2+ | Cl- | SO42- |
| S-1 | 6.8 | 1803 | 258 | 8.2 | 192 | 86 | 1.6 | 87 | 74 | 190 | 38.6 |
| S-2 | 7.1 | 1271 | 324 | 6.3 | 222 | 98 | 1.8 | 108 | 78 | 257 | 46.8 |
| S-3 | 7.1 | 1189 | 367 | 7.4 | 204 | 85 | 2.8 | 97 | 74 | 220 | 58.2 |
| S-4 | 7.9 | 3750 | 791 | 3.2 | 723 | 356 | 6.8 | 192 | 208 | 307 | 94.3 |
(All Values in mg/L except pH and EC)
Total Dissolved Solids (TDS) of the sample analyzed varies from 192 mg/L to 723 mg/L. Among these samples; S-4 has its TDS value above permissible limits. The higher value of TDS could be due to low water levels within aquifers and sediment effect11. The trend of TDS variations is shown in Fig. 2. The consumption of water having high dissolved solids and hardness may cause harmful effect like kidney stone formation and other related diseases.
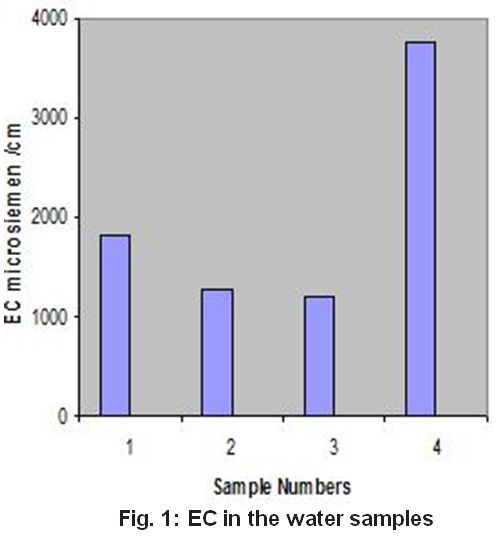 |
Figure 1: EC in the Water Samples Click here to view figure |
Dissolved Oxygen (DO) in the water is essential to aquatic life. It is important pollution parameter in water assessment and reflects the physical and biological processes prevailing in the water. The DO of sample S-4 is below the permissible limits indicating heavy contamination due to organic matters.
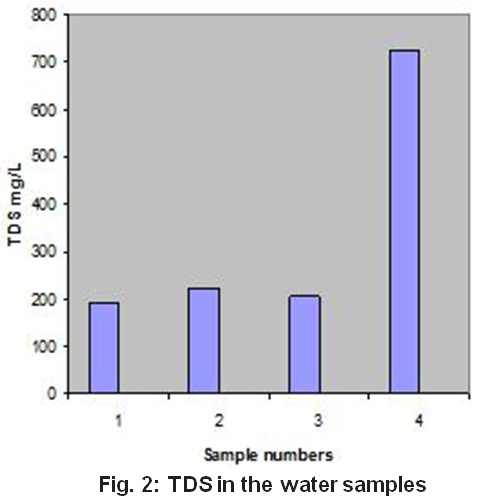 |
Figure 2: TDS in the Water Samples Click here to view figure |
The alkalinity in water is due to carbonates, bicarbonates and hydroxides. The alkalinity values ranged between 475 mg/L to 622 mg/L and found to be exceeds the permissible limits.
The Total Hardness (TH) of water samples analyzed varies from 258 mg/L to 791 mg/L which is higher than the permissible limits. The calcium content varies from 87 mg/L to 92 mg/L except for sample S-4 which is within the permissible limits.
The concentration of sodium and potassium were found within the permissible limits.
The chloride concentration of the water samples analyzed shows variation. The values for sample S-2 and S-4 are quite above the permissible limits; further chloride imparts salty taste to the water for the people who are not accustomed to high chloride concentration in water are subjected to laxative effect.
The Sulphate content of the water samples analyzed was found to be within the permissible limits. These values vary from 38.6 to 94.3 mg/L.
Conclusion
The results of the present work imply that the quality of the water samples S-1 and S-3 have good potability with all physico-chemical parameters below the permissible limits. However water samples S-2 and S-4 have inferior potability due to effluents from house hold industries and sewage. Among these water samples S-4 is highly polluted and unfit for drinking purpose and hence some suggestions are made from our side that there should be proper development of drainage network, conservation of ground water resource by means of executing proper management plan of ground water reservoir and launching of awareness program among the peoples about the health while utilizing such ground water for drinking purpose.
References
-
Singh K P, Malik A and Sinha S, Analytica Chimica Acta, 538, 355 (2005),
-
Palanisami P N,Geeta G A.,sujatha M,Sivakumar P and Karunakaran K, E Journal of Chemistry, 4(3): 434 (2007).
-
Manzoor S, Munir shah H, Shaheen N and Khalique A, J Hazard Mat. 137(11): 3(2006).
-
APHA, Standard Methods for examination of Water and Wastewater, Washington D C 18th Ed 1992.
-
Manivasakam N, Physicochemical Examination of Water, Sewage and Industrial Effluents: 5th Ed. Pragati Prakashan, Meerut (2005).
-
Kotaiah B and Kumarswamy N, Environmental Engineering Laboratory Manual,1stEd Chaotar Publishing House Pvt, Ltd.India.
-
WHO, International Standards for Drinking Water, Geneva: (1971).
-
ISI, International Standards of Water Quality for Drinking Water IS: 10500, 1983.
-
ICMR, Manual Standards of Water Quality for Drinking Water Supplies, 2nd Ed, Indian Council of Medicinal Research, New Delhi pp21 (1973).
-
Freeda Gnana Rani D, J Environ Sci Engg., 48(3): 199 (2003).
-
Damodaram T and Suresh S, Pollut Res., 24(1): 217(2005).






