Ground Water Hydrology in rural parts of Muzaffarnager District, Uttar Pradesh, India
Wequar Ahmad Siddiqui1 * and Mohd Waseem1
1
Department of Applied Sciences and Humanities,
Faculty of Engineering and Technology,
Jamia Millia Isalamia,
New Delhi,
110025
India
DOI: http://dx.doi.org/10.12944/CWE.6.2.11
The study area is a Krishna micro watershed in the central Ganga plain which is a highly fertile track of western Uttar Pradesh. The sugarcane and wheat are major crops of the area. Due to pressure of human activity, urbanization and degraded gradually the pure, safe, healthy and order less drinking water is a matter of deep concern. There are many pollutants in ground water due to seepage viz organic and inorganic pollutants, heavy metals, pesticides, fluorides etc. In Muzaffarnager district UP. India, mostly villages are affected with high TDS concentration in ground water. The water samples were analysed for pH, hardness, TDS, Chloride, Sulphlate, Nitrate, DO, COD ,Conductivity and some important heavy metals like, Copper (Cu), Chromium (Cr), Cadmium (Cd), Cobalt (Co) Zinc (Zn) and Nickel (Ni).
The results were compared with BIS standard for drinking water. Subsequent analysis of water samples show that the results are within the maximum permissible limits. Therefore the ground water of rural area of Muzaffarnager district is suitable for drinking, bathing and irrigation purposes.
Copy the following to cite this article:
Siddiqui WA, Waseem M. Ground Water Hydrology in Rural Parts of Muzaffarnager District,Uttar Pradesh, India. Curr World Environ 2011:6;275-278 DOI:http://dx.doi.org/10.12944/CWE.6.2.11
Copy the following to cite this URL:
Siddiqui WA, Waseem M. Ground Water Hydrology in Rural Parts of Muzaffarnager District,Uttar Pradesh, India. Curr World Environ [serial online] 2011;6:275-278. Available from: http://www.cwejournal.org/?p=1402
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-11-30 |
|---|---|
| Accepted: | 2011-12-31 |
Introduction
The study area spreads in an area of 650 Km part of Krishna River. It is one of the densely cultivated tracts and serves as leading producer of number of crops especially sugarcane, paddy and wheat. The high stress on ground due to pumpage of large quantities of ground water for irrigation has threatened the sustainability of agricultural development. Therefore, it is necessary to adopt exploitation of ground water to its availability accordingly a refined quantitative evaluation of ground water resources of an area or basin becomes an essential prerequisite for its management.
The study are, falling in the Muzaffarnager district of Uttar Pradesh, lies between latitudes 29O’N and longitudes 77 O 07`E and 78 O 22`E. The area covered during this study is about 650 sq km. The study area lies in between the river Krishna on the east and river yamuna on the west. The drainage is mainly controlled by the river Krishna and yamuna, which are flowing from north to south. River Yamuna from upstream to side, for about 3 km behaves as influent stream. The area constitutes two blocks of Muzaffarnager district namely Shamli and Kairana. In the kharif season, during which mainly wheat and paddy are sown the water level drops mainly due to ground water pumping for irrigation. More over, sugarcane is sown round the year and its irrigation further drops the ground water level. The area enjoys a sub-tropical climate with very hot summer and moderately cold winter. The maximum temperature recorded during summer 46 O C (in the month of Jun) and the lowest temperature recorded in January is 6 OC. The average annual rainfall in the area is 670.18 mm of which 80% is received as result of southwest monsoon during the month of July to September.
Material and Methods
Twenty samples were collected from hand pumps, tube wells from villages, temples, nearby power house, park, bus stand, juggi cluster and different factories. These samples were analysed for various physicochemical parameters and some heavy metals. The procedure followed for the analysis was from standard method (APHA).8 All water samples were collected in the polypropylene bottles which were properly washed with 20 % nitric acid and subsequently with double distilled water. Chemicals used in the analysis were Qualigens ExcelaR grade acids Indicators are of super pure grade.
Atomic Absorption Spectrophotometer (model 3100) Perkin Elemer USA was used for determination of heavy metals. Hydrogen ion concentration (pH), total dissolved solids (TDS) and conductivity were measured using pH, TDS and Conductivity meter respectively. Total Alkalinity (TA) was estimated titrimetrically using hydrochloric acid. Total hardness (TH) and Calcium (Ca2+) were analyzed titrimetrically using standard Ethylene Diaminetetraacetic acid disodium salt (EDTA). Magnesium (Mg2+) was determined taking the difference between total hardness (TH) and (Ca2+) values. Chloride (Cl-) were estimated using standard silver nitrate (AgNO3) while Sulphlate (SO42-) analyzed with the help of spectrophotomer. Dissolved Oxygen (DO) and (BOD) biological oxygen demand were analyzed titrimetrically using standard sodium thiosulphate (Na2S2O3) solution.
Results and Discussion
The results of physicochemical studies are summarized in the Table-1 where as the amount of heavy metals in different samples is listed in Table-2. In the present study, temperature varied from 26- 32 OC with day temperature 25 OC (min) to 37 OC (max).
pH is considered as an important ecological factor, as piece of information in many types of geometrical equilibrium or solubility calculation pH is an important parameter in water body since most of the aquatic organism are adapted to an average pH and do not withstand about changes. The pH values fluctuated between (7.1-7.9) Table -1. The limit of pH value for drinking water is specified as (6.5 to 8.5)
The conductivity was found to be varying between 0.71–4.50 (mS/cm) as shown in the Table-1.
Total dissolved solids (TDS) ranged between (350-1431) mg/l. The maximum permissible level is 2000 mg/L A large number of solids are found dissolved in natural water the common ones are carbonates, bicarbonate, chlorides, Sulphlate, phosphates and nitrates of calcium magnesium sodium, potassium iron etc. In other words TDS is sum of the cations and anions concentration. A high contents of dissolved solids elevates the density of water, influences solubility of gases (like oxygen) reduces utility of water for drinking irrigation and industrial purposes.
Total Alkalinity values were found to be varying from (260 – 850) ppm. Alkalinity of all water samples were found to be above the maximum desirable limit of 200 ppm. The values of alkalinity in water provide an idea of natural salts present in water. The cause of alkalinity is the minerals which dissolve in water from soil. The various ionic species that contribute to alkalinity include carbonate bicarbonate, hydroxide, phosphate and organic acids. High alkalinity of water reduces its industrial use.
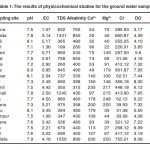 |
Table 1: The results of physicochemical studies for the ground water sample Click here to View table |
The summation of calcium and magnesium hardness is regarded as the total hardness of water. In the present investigation it has been observed that the calcium concentration is at least two folds greater than that of magnesium. Each of the samples has registered high values of calcium hardness (30 – 235 mg/l) while magnesium hardness is in thee range of (22 – 220 mg/l) as shown in the table No.1.
Chlorides are important in detecting the contamination of ground water. Appreciably high values of chloride ions were observed in drinking water sample Nos. 2, 8 and 20. The values are 660.87, 931.87 at 755.21 respectively. However the amount of chloride ions in rest of the samples was with in the desirable limits. High chloride ion contents in water sample may affect the taste of drinking water. DO and BOD ranged from (3.17 0 8.08 ppm) and (0.55 – 33.09 ppm) respectively at different sampling stations. BOD is the amount of DO required to stabilize the biodegradable organic matter by micro-organism of water under aerobic conditions. Higher BOD values may attribute to the stagnations of water body leading to the absence of salt purification cycle. Increase of COD values is due to pollution of input zones.
Excess amount of Sulphlate in water sample has cathartic effect on human health. The highest value was recorded at Baghra (77.7 ppm) and the lowest (11.6 ppm) at Lilon. The desirable limits of Sulphlate are 250 ppm as BIS standard.
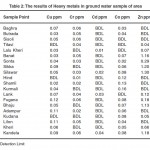 |
Table 2: The results of Heavy metals in ground water sample of area Click here to View table |
Results of heavy metals detection (Table-2) shows that Copper (Cu) which is an essential element in human metabolism and is considered to be non toxic up to 0.05 mg/l concentration in drinking water. In the present study of the drinking water the amount of Cu ranged from (0.011 to 0.03) mg/l which is with in the desirable limit. The amount of Cadmium (Cd) in this study was in found the range from 0.01 to 0.04 which properly lies within the permissible limit.
In the present study the amount of Nickel (Ni) and Cobalt (Co) is ranging from 0.02 to 0.16 and 0.03 to 0.13 mg/l, respectively which are with in permissible limit. Zinc (Zn) is also an essential trace element found in potable water sample. Zinc concentration was found to ring from 0.04 to 1.30 mg/l. Observed Zn concentration values were much lower than the permissible limit of 5 mg/l. The ground water therefore, was clearly Zn-deficient. Zinc deficiency may leads to dwarfism, dermatitis and loss of taste.
Conclusion
The study has led us to conclude that the ground that ground water samples of the studied site are acceptable for drinking purposes It has been observed that the amounts of almost all the indicative physicochemical parameters fall with in the permissible limits of drinking of BIS. Studies on heavy metals indicate that the amount of Zn in all the samples is below the permissible limit, however other important heavy metals present accordingly with the permissible limit. It is concluded therefore that the ground water of the entire sampling site is safe for drinking and other domestic purposes.
References
1. R. Shayamala M. Shanti and P. Lalitha ,Physicochemical Analysis of Borewell water samples of Telunguppalayam area in coimbatore District, Tamilnadu, India E Journal of chemistry vol. 4 pp 924 -929.
2. Mohammad Muqtada Ali Kham, Rashid Umar and Habibah Lateh, Study of trace elements in ground water of western Utter Pradesh, India , Scientific Research and Essays Vol 5(20) pp 3175-3182. Oct 2010.
3. Water Facts- Water and rivers commission of Western Australia, December 1998.
4. Kumar MD, Tushaar S, The Hindu Survey of the Environment, 2004, 7-9, 11-12.
5. Sanjeev L, Dogra TD, Bhardwaj DN, Sharma RK, Murty OP and Aarti V, Indian J Clin Biochem., 2004, 19(2) 135-140.
6. Preventing Groundwater Contamination, Fact sheet, Michigan Department of Environmental Quality Environ Sci Services Division, 1994.
7. Rao NS, Hydrological Sci. J/J des Sci Hydrologiques, 2003, 48(5), 835-847.
8. Standard methods for the examination of water and waste water, 1985, 16th ed., APHA, AWWA and WPCF Inc. New York.







