Assessment of Heavy Metals in Water, Fish and Sediments from UKE Stream, Nasarawa State, Nigeria
O. D. Opaluwa1 * , M. O. Aremu1 , L.O L.OGBO2 , J. I.magaji2 , I. E.Odiba3 and E. R. Ekpo1 *
1
Department of Chemistry,
Nasarawa State University,
Keffi,
Nigeria
2
Department of Geography,
Nasarawa State University,
Keffi,
Nigeria
3
Department of Geology and Mining,
Nasarawa State University,
Keffi,
Nigeria
DOI: http://dx.doi.org/10.12944/CWE.7.2.04
Copy the following to cite this article:
Opaluwa O.D, Aremu M.O, Ogbo L.O, Magaji J.I, Odiba I.E, Ekpo E.R. Assessment of Heavy Metals in Water, Fish and Sediments from UKE Stream, Nasarawa State, Nigeria. Curr World Environ 2012;7(2):213-220 DOI:http://dx.doi.org/10.12944/CWE.7.2.04
Copy the following to cite this URL:
Opaluwa O.D, Aremu M.O, Ogbo L.O, Magaji J.I, Odiba I.E, Ekpo E.R. Assessment of Heavy Metals in Water, Fish and Sediments from UKE Stream, Nasarawa State, Nigeria. Curr World Environ 2012;7(2):213-220. Available from: http://www.cwejournal.org/?p=2767
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2012-07-12 |
|---|---|
| Accepted: | 2012-09-17 |
Over the last few decades, there has been growing interest in determining heavy metal levels in the marine environment and attention was drawn to the measurement of contamination levels in public food supplied, particularly fish.1-3 Although heavy metal is a loosely defined term,4 it is widely recognized and usually applied to the wide spread contaminants of terrestrial and fresh water ecosystems. Some examples of heavy metal include lead, zinc, cadmium, copper and manganese. Many of these heavy metals are toxic to organisms at low concentration.5-6
The concentration of metals in bio-available form is not necessarily proportional to the total concentration of the metal. The concentration of various elements in the air, water and land may be increased beyond their natural level due to the agricultural, domestic and industrial effluents. These substances are described as “contaminants” when discharged to the environment.6 In water, insoluble heavy metals may be bound to small silt particles. Metals and other fluvial contaminants in suspension or solution, do simply flow down the stream, they form complexes with other compounds settle to the bottom and ingested by plants and animals or adsorbed to sediments.7 Consequently, aquatic organisms may acquire heavy metals in body directly from the water via gills or food chain mechanisms.8
Work has been presented on heavy metal concentrations in water, sediments and fishes from Nasarawa and Antau streams in Nasarawa State to ascertain the extent of heavy metal pollution in these aquatic ecosystems and their eventual uptake by aquatic organisms. Different parts of fish (head, gills, intestine and flesh) were used and by drying these parts of fish and the sediment samples and employing the method of wet digestion and AAS, the level of metals in these parts of fish and the sediment were determined. The level of metals in water was also determined using AAS after pretreatment. The results showed the presence of metals determined in all the samples but were below the deleterious level.5, 9
Water and sediments are commonly used as indicators for the state of pollution of aquatic ecosystem.10 Uke stream runs through the centre of the town and the water from this stream serves domestic purposes as well as irrigation farming and aquatic organisms (fish) from this water body is one the major sources of protein for the populace of this location. However, the stream also serves as points of discharge for domestic wastes in some areas along the length of the stream and runoffs from agricultural lands always flow into the stream at different points.
Aquatic animals (including fish) bio-accumulate heavy metals in considerable amount in tissues over a long time and the dependence of the populace in this area on this water body for domestic water supply and its aquatic organisms (fish) as source of protein makes it imperative to assess the level of heavy metals in this aquatic ecosystem in view of the health implications that cut across the food strata.
This research reports the level of Pb, Zn, Fe, Cd, Cu and Mn in parts of fish caught from Uke stream as well as the stream water and sediments in order to ascertain the relationship between bioaccumulation of these metals in the aquatic organisms (fish) with the distribution and concentrations of these metals in the stream water and sediments. This is aimed at ensuring the safety of this ecosystem for the benefits of the residents of Uke and its environs.
Materials and Methods
Collection of Samples
Water samples were collected using plastic containers to fetch water below the surface at designated points, mixed properly and stored in a plastic container rinsed with 0.01N nitric acid and kept in deep freezer prior to the time of analysis.11 The sediment samples were collected by scooping with a plastic spoon from the points where the water samples were taken, air dried and kept awaiting analysis. The samples of available fish species (Clarias gariepinus and Synodontis schall) in the stream were purchased from fishermen at the stream site. They were properly and carefully washed and stored at 40C pending analysis. These samples were all collected at7.00 local time while the temperature (28ºC) of the water was taken at the point of collection.
Sample Treatment
Five (5.0) cm3 of concentrated hydrochloric acid were added to 250.0 cm3 of water sample and evaporated to 25.0 cm3. The concentrate was transferred to a 50.0 cm3 standard flask and diluted to the mark with de-ionized water.11 5.0 g of prepared sediment sample was digested with 15.0 cm3 nitric acid, 20.0 cm3 perchloric acid and 15.0 cm3 hydrofluoric acid and placed on a hot plate for 3h. On cooling, the digest was filtered into a 100.0 cm3 volumetric flask and made up to the mark with distilled water.12 Different body parts of the fish (Head, gills, intestine and flesh) were dried in the oven at 1050C until constant weight is obtained and blended. 2.0 g of the blended fish parts were weighed and digested using the approved method.13
Mineral Analysis
Lead, zinc, copper, iron, manganese, cadmium and mercury were determined in samples of fish body parts of Clarias gariepinus and Synodontis schall, water and sediment using computer controlled Atomic Absorption Spectrophotometer (AAS VGB 210 System). The instrument setting and operational conditions were done in accordance with the manufacturers’ specifications. All determinations were in triplicates.
Statistical Analysis
The results obtained were subjected to statistical evaluation. Parameters evaluated were grand mean, standard deviation (SD) and coefficient of variation (CV %).
Results and Discussion
Table-1 shows the mean metal concentrations, grand mean, standard deviation and coefficient of variation percent of water and sediments. The mean concentration of metals determined in the water samples ranged from 0.023 – 7.51 mg/L and for sediments the range was 0.095 – 8.78 mg/g. The metals determined were Pb, Zn, Fe, Cd, Cu, Mn and Hg with mean concentrations of 0.040, 3.19, 7.51, 0.023, 0.95, 0.51 (mg/l) in water and 0.095, 4.79, 8.78, 0.035, 1.34, 0.24, and (mg/g) in sediment respectively with Hg not detected in both water and sediment samples. The concentrations of Fe, Zn, in both samples and Cu in sediment were high (> 1.0 mg/L). The concentration of Fe being highest in sediment agrees with the result of the report of heavy metals in sediments of Rafin Mallam stream10 but its value in water, also highest is high than the value recorded in the report of heavy metals in water and fish from River Antau.5 However, the concentration of Fe in sediments and water to an extent is determined by the nature of soil along the stream banks14 from where it is leached into the water body and sediments. The values of Zn recorded are lower than the ones obtained from the results of the report of heavy metals in water, sediments and fish from River Nasarawa and for Cu, its concentration is the same for water and higher for sediment than that obtained for River Nasarawa.9 Zinc is widely used for making paints, dyes, rubber, wood preservatives and through wares and tears; zinc from this sources is discharged into the environment. Although zinc is required by plants and animals for normal growth, higher concentration of it is toxic.15
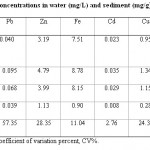 |
Table 1: Mean metal concentrations in water (mg/L) and sediment (mg/g) Click here to View table |
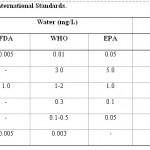 |
Table 2: National and International Standards Click here to View table |
The concentrations of Cd and Pb obtained are lower for water and higher for sediments from the work on a water body used for irrigation in Keffi.10 The concentration of Mn in sediment is lower and higher in water than those obtained from the work carried out on River Tammah.11 Cu, Pb and Mn are some of the metals that get into aquatic ecosystem from runoffs from agricultural lands as a result of the use of agrochemicals containing heavy metals such as Cu, Mg, Mn, Pb and Zn.16 The probable sources of Cd in surface water includes leaching from Ni – Cd batteries, run off from agricultural soils where phosphate fertilizers are used and other wastes.17,18
All the metals determined were above the World Health Organization (WHO) safety standards, Food and Drugs Administration (FDA) Table-2, and the United States Environment Protection Agency (USEPA) maxima19 except for copper in water which is within the range recommended by W.H.O higher than the ranges recommended by other regulatory bodies. The level of zinc in sediment is however within the range recommended by EPA.20 Iron though high above WHO safety standard, it is still safe because it has benefits to organism though in very high concentration leads to conjunctivitis, chroiditis and retinitis if it is in contact and remains in the tissue but Cd is a toxic metal and has no metabolic benefits to human and aquatic biota.21 Its presence in any compartment of the aquatic ecosystem indicates contamination.
The high level of these metals in both the water and sediment samples are as a result of the runoffs during the rainy season from agricultural fields and the dumping of domestic wastes in the water body at different points along the length of the stream as they are known to contain heavy metals such as As, Cd, Co, Cu, Fe, Hg, Mn, Pb, Ni and Zn which will eventually end up in this aquatic ecosystem.22
Among all these metals determined iron has the highest concentration with average of 8.78 mg/g and 7.51 mg/l in sediment and water, respectively followed by Zn with 4.79 mg/g (Sediment) and 3.19 mg/l (Water) while Cd has the lowest concentration of 0.035 mg/g and 0.023 mg/l in the sediment and water respectively. These results are in agreement with the result of in which Cd was found to have the lowest concentration in both sediment and water. Going by the calculated coefficient of variation percent (CV %) when the levels of metals in water and sediment were compared the variability was highest in lead while cadmium was the least varied.
Table-3 and Table-4 show mean metal concentrations in the body parts (head, gills, intestine and the flesh) of African catfish, Clarias garipienus and Synodontis schall respectively. The presence of these metals analysed in the body parts of fish serves as an indicator for the extent of heavy metal pollution of the water body from where these aquatic organisms (fish) are obtained.9 Also the presence of most of the metals determined in the fish parts agrees with the results of the report of the level of heavy metals in aquatic organism from different water bodies23,24 which showed that aquatic animals, fish, inclusive bio-accumulate heavy metals in considerably amount, and because these metals are not bio-degradable, the metal tend to stay in the fish tissues for a very long time which upon consumption of these fish, the heavy metals get transferred to man, leading to heavy metal poisoning in man especially if present in higher concentrations. In both species of the fish zinc presented the highest concentrations followed by iron in the various parts studied and this is relative to the concentrations of these metals observed in both water and sediment samples.
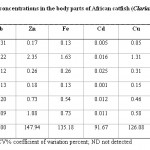 |
Table 3: Mean metal concentrations in the body parts of African catfish (Clarias garipienus), mg/g Click here to View table |
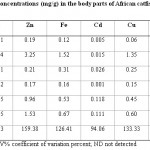 |
Table 4: Mean metal concentrations (mg/g) in the body parts of African catfish (Synodontis schall) Click here to View table |
Copper was the next metal after zinc and iron with concentration range of 0.05 – 1.35 mg/g in the various parts of both species of the fish studied. Lead, cadmium and manganese showed different distribution among the various parts of fish. Mercury was not detected in any part of both species just as it was neither detected in water nor sediment samples. Zinc showed the highest variability of 147.94 and 159.38 % in Clarias garipienus and Synodontis schall respectively with lead having the least in both cases. This results agrees with that obtained for the analysis of the levels of metals in organs of Clarias lazera from river Nasarawa.9
Most of the metals had highest concentrations either in the head part (lead and manganese) or the gills part (zinc, iron and copper). This is as result of the fact that the gills helps in respiration and filtration of water.24 Relatively high concentrations of some of the metals were found in the intestine since the intestine is part of the visceral muscles which concentrates toxic metals.25 Zinc and copper are mineral elements which are essential metals and play vital role in enzyme activity and iron is very important in heamoglobin formation. Lead and cadmium are toxic at very low concentration and have no known functions in biochemical processes. Sources of cadmium include wastes from cadmium- based batteries, incinerators and runoff from agricultural soils where phosphate fertilizers are used since cadmium is a common impurity in phosphate fertilizers.18 Lead is mainly from storage batteries, type metal and anti-knock compound in petrol.26 The levels of all the metals determined were however below the concentrations recommended by regulatory bodies.21
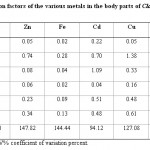 |
Table 5: Bioconcetration factors of the various metals in the body parts of Clarias garipienus Click here to View table |
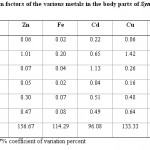 |
Table 6: Bioconcentration factors of the various metals in the body parts of Synodontis schall Click here to View table |
Table-5 and Tables-6 show the bioconcentration factors for Clarias garipienus and Synodontis schall, respectively. Most of the values obtained for the various fish parts were relatively low (< 1) which showed there was no biological magnification of metal concentration in fish samples except for cadmium (in the intestine) and copper (in gills) for Clarias garipienus and zinc (in gills), cadmium (in the intestine) and copper (in gills) for Synodontis schall. Order of bioconcentration in the various body parts of Clarias garipienus is Zn > Fe > Cu > Cd > Pb > Mn while that of Synodontis schall is Zn > Cu > Fe > Cd > Mn > Pb. Zn showed the widest variation in both species of fish while the least variations were recorded in Mn and Pb for Clarias garipienus and Synodontis schall, respectively. The levels of metals in the body parts of the two species of catfish were lower than that of the water or sediment. However the presence of metals in the two fishes biochemically showed that fish is relatively dependent on the levels of metals available in aquatic ecosystem.
Conclusion
This research has presented data on the levels of heavy metals in sediments, water and various body parts of two species of catfish from Uke stream in Uke town, Nasarawa State. Although the results obtained does not show any form of danger posed to consumers of sea foods and water from this stream but the possibility of deleterious effects after long period cannot be ruled out. This is as a result of the fact that this water body serves as the receptor for domestic wastes as well as runoff from agricultural lands where phosphate fertilizers and other agrochemicals are frequently used. There is therefore the need for continual assessment of the level of pollution of this stream with metals from the mentioned sources with a view to reducing the level of pollution via education and public enlightenment.
References
- Kalay, M; Aly,O. and Canil,M., Heavy metal concentrations in fish tissue from the Northeast Mediterranean Sea, Bullentin of Environ. Contamination and Toxicity, 63, 673-671 (1999). http://dx.doi.org/10.1007/s001289901033
- Rose, J., Hutcheson, M.S., West, C.R .and Pancorbo, O., “Fish Mercury Distribution in Massachusetts, USA Lakes”, Environ. Toxicology and Chem., 18(7), 1370-1379 (1999). http://dx.doi.org/10.1002/etc.5620180705
- Tariq, J., Jaffa, M. and Ashraf, M., “Heavy Metal concentrations in fish, shrimp, seaweed, sediment and water from Arabian Sea, Pakistan”, Marine Pollution Bulletin, 26(11), 644-647 (1993). http://dx.doi.org/10.1016/0025-326X(93)90504-D
- Duffus, J. H., “Heavy Metal” – A meaningless term. Pure and Applied chemistry, 74, 793-807 (2002). http://dx.doi.org/10.1351/pac200274050793
- Galloway, J.N, Thornton, J.D, Norton, S.A, Volchok, H.L and MCclean H.L, Trace Metals in Atmospheric depositions, A review and assessment. Atmospheric Environment, 16, 1677-1700 (1982). http://dx.doi.org/10.1016/0004-6981(82)90262-1
- Madu, P.C., Tagwoi, J.T. and Babalola, F., A study of heavy metal pollution of River Antau, Keffi, Nasarawa State, Nigeria, India J. of Multi. Res., 4 (1), 8-18 (2008).
- Odiete, W.O., Environmental Physiology of Animals and Pollution, 1st ed. Diversified Resources Limited, Lagos.1-end (1999).
- Collison, C. and Shrimp, N.F., Trace elements in bottom sediments from Upper Peria Lake. Middle Illinois River. Illinois Geo. Survey and Environ. Geology Note, 56, 21 (1972).
- Huckabee, J.W and Blaylock, B.G., Transfer of mercury and cadmium from terrestrial to aquatic ecosystem. Environmental sciences Division, Oak Ridge National Laboratory Oak Ridge, Tennesse, 55 (1972).
- Aremu, M.O., Atolaiye, B.O., Shagye, D., and Moumouni, A., Determination of trace metals in Tilapia zilli and Clarias lazera fishes associated with water and soil sediment from River Nasarawa in Nasarawa State, Nigeria, India J. Multi. Res., 3(1), 159-168 (2007).
- Opaluwa, O.D. and Umar, M.A., Level of heavy metals in vegetables grown on irrigated farmland, Bull. of Pure and Applied Sci., 29C (1), 39-55 (2010).
- Atolaye, B. O, Aremu, M.O., Shagye, D. and Pennap, G.R. I., Distribution and concentration of some mineral elements in soil sediment, ambient water and body parts of Claria gariepinus and Tilapia queneensis fishes in stream Tammah, Nasarawa State Nigeria, Curr World Environ., 1(2), 95-100 (2006).
- Adekenya B., Variation of metal pollutants with depths, Techforum, An Interdisci., J. 2, 3,82-97 (1998).
- Ibok, U.J; Udosen, E.D and Udoidiong, O.M., Heavy Metals in Fishes from Streams in Ikot Ekpene Area of Nigeria, Nigeria J. Tech. Res., 1, 61-68 (1989).
- Osakwe, S. A. and Peretiemo-Clarke, B.O., Evaluation of heavy metals in sediments from River Ethiope, Delta State, Nigeria. 31st CSN Conference paper, 611-613 (2008).
- Umar, M.A. and Opaluwa, O.D., Evaluation of heavy metals in sediments of Rafin Mallam stream in Keffi, Nasarawa State, Intl. J. Chem., 20 (2), 99-103 (2010).
- Pate, K.P., Pandy, R.M. and George, L., Heavy metal content of different effluents water around major industrial cities of Guryurat, J. of Indian Society of Soil Sci., 59 (1),89-94 (2001).
- Hutton, M. and Symon, C., The quantities of cadmium, lead, mercury and arsenic entering the U.K environment from human activities, Science of the total environment, 59, 129-150 (1986). http://dx.doi.org/10.1016/0048-9697(86)90018-5
- Stoeppler, M., Cadmium. In: Merian E (ed) Metals and their compounds in the environment: Occurrence, analysis and biological relevance. VCH. New York, 803-851 (1999).
- United States environmental protection Agency (USEPA), Quality Criteria for Water. United States Environment Protection Agency Office of Water Regulations and Standards, Washington DC, 20460, 1986a.
- Environmental Protection Agency EPA, (1976): “Quality Criteria for Water”. Washington, 440 (9), 76 – 123 (1986a).
- Woodworth, J.C and Pascoe, V., Cadmium toxicity to rainbow trout, salmon gairdneri Richardson, A Study of Eggs and Alevins, J. Fish. Biol., 21, 47-57 (1982). http://dx.doi.org/10.1111/j.1095-8649.1982.tb02822.x
- Oluyemi, E.A.; Fenyuit, G.J; Oyekunle, J.A.O. and Ogunfowokan, A.O., Seasonal variations in heavy metal concentrations in soil and some selected crops at a landfill in Nigeria, African J. of Sci. and Tech., 2(5), 89-96 (2008).
- Kemdrin, E.C., Trace metal contents of microbenthos of two city reservoirs in Jos, Plateau in relation to their feeding functional groups, N.J.T.E., 14(1), 42-44 (1979).
- Etuk, E.U.I and Mbonu, C.O., Comparison of trace and toxic metal contaminants in Perionkle from Qua-Iboe River (Ibeno) and Cross River (Oron), Proceeding of the 23rd Annual Conference of the Nigeria Institute of Food Science and technology held at Abuja Oct. 25-27th, (1999).
- Ayejuyo, O.O., Olowu, R.A., Megwa, K.C., Denloye, A.A.B. and Owodehinde, F. G., Trace metals in Clarias lazera, water and sediments from Majidun River, Ikorodu, Nigeria Res Commun. Fishries, 1, 27-31 (2003).
- Atta, M.B; El-Sebaie, L.A.; Naoman, M.A and Kassab, H.F., The effect of cooking on the contents of heavy metals in fish (Tilapia nilotica). Food Chem. 58, 1-4 (1997). http://dx.doi.org/10.1016/0308-8146(95)00205-7
- Crossby, N.T. Determination of metals in Food. A Review. Analyst, 102, 225-268 (1997). http://dx.doi.org/10.1039/an9770200225







