Studies in the applicability of organic polymer for pretreatment of industrial waste
Dhananjay Dwivedi1 , K. Yadav2 and Vijay R.Chourey3 *
1
P.M.B. Gujarati Sc. College,
Indore,
India
2
Government Autonomous Holkar Science College,
Indore,
452 001
India
3
2603-E Sudama Nagar,
Indore,
452 009
India
DOI: http://dx.doi.org/10.12944/CWE.7.2.18
Copy the following to cite this article:
Dwivedi D, Yadav K, Chourey V.R. Studies in the Applicability of Organic Polymer for Pretreatment of Industrial Waste. Curr World Environ 2012;7(2):305-308 DOI:http://dx.doi.org/10.12944/CWE.7.2.18
Copy the following to cite this URL:
Dwivedi D, Yadav K, Chourey V.R. Studies in the Applicability of Organic Polymer for Pretreatment of Industrial Waste. Curr World Environ 2012;7(2):305-308. Available from: http://www.cwejournal.org/?p=2909
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2012-07-12 |
|---|---|
| Accepted: | 2012-09-17 |
waste effluents coming out from the Process industries creating lot of environmental problems. Waste effluent from metal plating, refining, battery and power source are found to have varying degree of contaminants with high toxicity.1 There are various methods and techniques available for treatment of toxic waste to maintain the toxic material below the prescribed disposable limit. However, these techniques suffer the load of physical and chemical pollutants. For the fastness of main treatment process and to increase the efficiency of full fledged treatment process a kind of prior treatment can be given to waste in form of flocculation and coagulation.
By pretreatment the size of impurities increases to such extent that they can be easily filtered out from the effluents. Generally in the pretreatment of electroplating waste many inorganic coagulants or flocculants are used but they have some disadvantages with them i.e. they may increase the unwanted ionic load. To avoid such type of unrequired ionic loading the organic polymers can be used as flocculation agents.2,3
Organic polymers or polyelectrolyte’s are water soluble. They may be synthetic or natural in origin like cellulose derivatives, starch product etc. They are nontoxic and their small dosing is required for the flocculation.4
Material and Methods
SS-120 is an anionic polymer with high molecular weight and in form of white granulated powder is used as flocculation agent. It was supplied by Thermax India Pvt. Ltd. Other chemicals as NiCl2, CuSO4, EDTA, ZnCl2, CuCl2 etc. were used of AR grade. All the solutions were prepared in doubly distilled water.
Experimental Procedure
The stock solution of organic polymer SS-120 was prepared as 1 mL of this stock solution to give 1mg/L of polyelectrolyte concentration when added to the 1 liter of test solution. For required dosing different mL of stock solution have been used. The mixture of required amount of polymer in distilled water was kept on the magnetic stirrer to get homogenous solution which should be viscous in nature. Ion containing test solutions were prepared by dissolving their required amount in distilled water.
Treatment Procedure
Required dosing of organic polymer from stock solution was added to definite amount of prepared waste solution. For complete and constant mixing it is continuously stirred for 25 minutes then kept for 10 minutes to get settled. The flocs formed by flocculation get separated by routine methods. The filtrate was tested for ions, TDS, DO and pH estimation. Ni+2, Cu+2 and Cl- were tested by complexometrically, iodometrically and argentometrically respectively.5,6
Results and Discussion
Experiments were performed and their findings and related discussions have been summarized below:
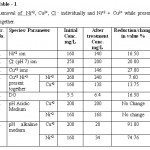 |
Table 1: Removal of Ni+2, Cu2+, Cl - individually and Ni+2 + Cu2+ while present together Click here to View table |
Experiment was also carried out to see the change in flocculation efficiency with changing pH. For this effluent with Cu+2 & Ni+2 ions were treated with organic polymer in two different pH ranges
b) at 7.3 pH (slightly alkaline range)
From the result it has been observed that in the acidic pH range there is no change in the initial concentration after treatment while in alkaline pH range change in concentration is observed. Ni+2 reduced from 168 mg/L to 34 mg/L & Cu2+ from 200 mg/L to 20 mg/L with reduction percentage of 80% & 90% respectively.
The observation exhibit that increase in the solubility of metal ions in acidic medium reduces the flock formation with polyelectrolyte, while in alkaline medium metal ions precipitated as hydroxide. Hydroxides are colloidal in nature and quickly form flocks with organic polymer and get settled. Hence high change in the value is obtained in alkaline range.
Concentration of dissolved oxygen was also tested in the untreated and treated (with biopolymer) electroplating waste effluent.
The results of this filtrate were recorded. There is an increase of DO by 16.5% in the biopolymer mixed effluent.
The studies has also been carried out for the determination of TDS (Total Dissolve Solids) at solution pH level in untreated Cu+2 and Ni+2 mixed effluents and in the effluent treated with biopolymer. The value of TDS reduced from 5260 mg/L to 1315 mg/L with 75% reduction. These studies also show that addition of aluminum sulphate in the treated effluent increases the TDS.
It has also been observed that if pH is adjusted to slightly alkaline side then TDS decreased from 5260 mg /L to 684 mg /L. The results are given in the table 2
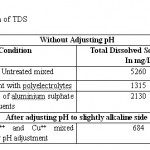 |
Table 2: Determination of TDS Click here to View table |
Observation reveals that SS-120 biopolymer reduces the TDS, while addition of Al2(SO4)3, increases the TDS.7 TDS decreases after adjusting pH to slightly alkaline side because of the metal hydroxide gets precipitated.
Work has also been performed on the effect of polyelectrolyte, Al2(SO4)3 and of pH on the sludge volume. Results are given in the table -3.
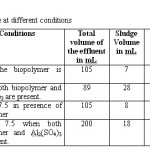 |
Table 3: Sludge volume at different conditions Click here to View table |
It shows that presence of biopolymer and alum affect the sludge volume in greater extent. Work has also been carried out to know the tendency of the biopolymer to form the flock with ions. For these solutions containing different metal and non metal ions were used. The results of visual observations are given in the table - 4
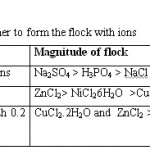 |
Table 4: Tendency of biopolymer to form the flock with ions Click here to View table |
These results confirmed that biopolymer SS-120 is more active than other metal ions as the flock of zinc formed has greater magnitude. In case of non-metal ions biopolymer exhibit more affinity towards the sulphate ion than chloride ions.
After the pretreatment of effluent, now it was treated by one of the selected full flagged treatment process. Here it was applied on ion exchange technique. In first step it was allowed to pass through the cationic exchanger for removal of Cu2+ and Ni2+ and then through the anionic exchanger to remove Cl- ions. With This experiment complete removal of anions and cations has been observed. The experiment was also monitored for any adverse effect of biopolymer on resin, but no such adverse effect was noticed.
It is assumed that the capacity of biopolymer to trap the colloidal pollutants is due to their long chain structure. The colloidal particles entangle or trapped with them and form compact and big size flocks.
The work performed, confirm that pre-treatment of industrial waste by use of biopolymer not only reduces various pollutants to remarkable extent but faster the main treatment process by quite and good margin.
The method is very much useful to minimize the load of impurities in the industrial effluents having high concentration of metal ions and anions by using biopolymer.
Acknowledgement
Authors are thankful to authority of PMB Gujarati Science College, Indore for providing research facilities.
References
- S.S. Rogers and P. Venema, Biopolymer, 82(3), 241 (2006). http://dx.doi.org/10.1002/bip.20483
- K. Nakanura and Rawarnura, Bul.Chem. Soc., Japan, 44, 330 (1971).
- K.E. Langford and J.E. Parkar, Analysis of electroplating and related solutions Robert Draper Ltd. Teddngton, (1971).
- N.V. Parathasardhy, Environ. Health, 11, 358, (1969).
- C.E. Van Hall and V.A. Stranger, Anal.Chem., 35, 315 (1963). http://dx.doi.org/10.1021/ac60196a014
- E.W. Meeker and E.C. Wagner, 2nd Eng. Chem. Anal. Ed. , 5,396 (1993). http://dx.doi.org/10.1021/ac50086a012
- M. Ali and N. Deo, Indian J. Environ. Protect., 12(3), 202 (1992).






