Environmental Prevalence of Pathogens in Different Drinking Water Resources in Makkah City (Kingdom of Saudi Arabia)
Abdullah A.Saati1 * and Hani S. Faidah2
1
Deparment of Community Medicine and Pilgrims Healthcare,
Faculty of Medicine,
Umm Al-Qura University,
Saudi Arabia
2
Deparment of Medical Microbiology,
Faculty of Medicine,
Umm Al-Qura University,
Saudi Arabia
DOI: http://dx.doi.org/10.12944/CWE.8.1.05
Copy the following to cite this article:
Saati A. A, Faidah H. S. Environmental Prevalence of Pathogens in Different Drinking Water Resources in Makkah City (Kingdom of Saudi Arabia). Curr World Environ 2013;8(1) DOI:http://dx.doi.org/10.12944/CWE.8.1.05
Copy the following to cite this URL:
Saati A. A, Faidah H. S. Environmental Prevalence of Pathogens in Different Drinking Water Resources in Makkah City (Kingdom of Saudi Arabia). Curr World Environ 2013;8(1). Available from: http://www.cwejournal.org/?p=3173
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-02-26 |
|---|---|
| Accepted: | 2013-03-24 |
Water is unsafe for human consumption when it contains pathogenic or disease-causing microorganisms. Pathogenic microorganisms (and their associated disease(s)) may include bacteria, such as Salmonella typhi (typhoid fever), Vibrio cholerae (cholera), Shigella (dysentery, shigellosis), and viruses, such as poliovirus or Hepatitis A virus and protozoa such as Giardia lamblia (giardiasis) or Cryptosporidium parvum (cryptosporidiosis). A major challenge for water suppliers is how to control and limit the risks from pathogens and disinfection by-products. It is important to provide protection from pathogens while simultaneously minimizing health risks to the population from disinfection by-products (EPA, 2011).
In addition to bacterial-related health risks, faecal contamination carries the increased risk of viral contamination of the water source. Although viruses cannot multiply in water, some may remain static. This health risk is elevated in treated water (i.e. chlorinated tanks) where the faecal indicators may be absent. Many viruses have been identified as key etiological agents in outbreaks of drinking water derived gastrointestinal illness in the United States and Netherlands (Leclerc et al., 2002). Some micro-fungi are known to be opportunistic human pathogens. Airborne spores are an important potential source of microfungi found in water storage reservoirs. It has also demonstrated conclusively that filamentous microfungi grow and sporulate on the inner surfaces of water pipe and in soft sediments within the water distribution system (Sammon et al., 2011). Worldwide, over one billion people lack access to an adequate water supply; more than twice as many lack basic sanitation (WHO/UNICEF, 2006). Unsafe water, inadequate sanitation, and insufficient hygiene account for an estimated 9.1 percent of the global burden of disease and 6.3 percent of all deaths, according to the World Health Organization (Prüss-Üstün et al., 2008).
This burden is disproportionately borne by children in developing countries, with water-related factors causing more than 20 percent of deaths of people under age 14. Nearly half of all people in developing countries have infections or diseases associated with inadequate water supply and sanitation (Bartram et al., 2005). The presence of E.coli in water is a strong indication of recent sewage or faecal contamination. Sewage may contain many types of disease-causing organisms. E. coli comes from human and animal waste. During rainfalls, snow melts, or other types of precipitation, E.coli may be washed into creeks, rivers, streams, lakes, or groundwater. When these waters are used as sources of drinking water and the water is not treated or inadequately treated, E.coli may end up in the drinking water (Health Canada, 2008).
Faecal coliforms and E.coli are bacteria whose presence indicates that the water may be contaminated with human or animal wastes. Microbes in these waters can cause short-term effects, such as diarrhea, cramps, nausea, headaches, or other symptoms. They may pose a special health risk for infants, young children, some of the elderly, and people with severely compromised immune systems (CDC, 2009). Some bacteria are ubiquitous in soil, water and on surfaces in contact with soil or water such as Pseudomonas aeruginosa which is an opportunistic pathogen. P.aeruginosa is an opportunistic pathogen.
It produces tissue-damaging toxins and causes urinary tract infections, respiratory system infections, central nervous system, endocarditis (P.aeruginosa infects heart valves establishes itself on the endocardium), dermatitis, soft tissue infections, bacteraemia, bone and joint infections, gastrointestinal infections and a variety of systemic infections, particularly in patients with severe burns and in cancer and AIDS patients who are immunosuppressed (EHA, 2012). Spread occurs from patient to patient on the hands of hospital personnel, by direct patient contact with contaminated reservoirs, and by the ingestion of contaminated foods and water (EHA, 2012).
The presence of faecal coliform in aquatic environments may indicate that the water has been contaminated with the faecal material of humans or animals. Faecal coliform bacteria can enter water bodies through direct discharge of waste from mammals and birds, from storm and agricultural runoff, and from human waste (Doyle and Erickson, 2006).
Pet wastes (cats, dogs) can contribute to faecal contamination of surface waters. Runoff from roads, parking lots, and yards can carry animal wastes to streams through storm sewers. Birds can be a significant source of faecal coliform bacteria. Birds (seagulls, geese, swans) can all elevate bacterial counts, especially in freshwater systems (wetland, rivers, lakes and ponds). Some waterborne pathogenic diseases that may coincide with faecal coliform contamination include ear infections, viral and bacterial gastroenteritis, dysentery, typhoid fever and hepatitis A. The presence of faecal coliform tends to affect humans more than it does aquatic creatures, though not exclusively (Walkerton, 2011).
Fungi are ubiquitous organisms that are widely distributed in nature. Several fungal genera have been shown to be allergenic, such as Aspergillus, Alternaria and Cladosporium (Black et al., 2000; Bowyer et al., 2006; Hedayati et al., 2007; Simon-Nobbe et al., 2006). Several studies have suggested an important role for waterborne fungi to endanger human health (Anaissie et al., 2001; 2003; Warris et al., 2001). Some of these studies have linked a genetic relationship between waterborne fungi and fungi isolated from clinical samples (Anaissie et al., 2001; 2003).
There are several species of Aspergillus which cause infection to the human especially Aspergillus fumigatus. Aspergillosis is opportunistic respiratory infection which causes about 40% of fatal nosocomial infections. Aspergillus spp infections are transmitted by water (Graybill, 2001). Drinking water quality is usually determined by its pathogenic bacterial content. However, the potential of water-borne spores as a source of nosocomial fungal infection is increasingly being recognized. Sammon et al. (2010) demonstrated that numerous microfungal genera, including those that contain species which are opportunistic human pathogens, populate a typical treated municipal water supply in sub-tropical Australia. Penicillium spp. contain more than 225 species confirming to certain morphological criteria (Pitt et al., 2000).
They can be isolated from the well water (Siqueira et.al, 2011). Penicillium marneffi is diamorphic, forming yeast-like cells in infected tissues, often they can found intracellular (Jolanta, 2005; Rajendran et al., 2006). In immunocomprimised people, P. marneffi considered as a common opportunistic pathogens which can cause systemic penicillosis in acquired immunodeficiency syndrome (AIDS) patients. Rodents are usual reservoirs for P. marneffi and may be involved in its transmission to humans (Cheesbrough, 2007). Pathogenic bacteria can occur in surface water in large numbers, either being excreted in faeces or occurring naturally in the environment.
Bacteria typically range in size between 0.5 and 2 micrometres. Disease-causing bacteria that can be transmitted by water include Vibrio cholerae, Salmonella sp, Campylobacter sp, Shigella sp, and Staphylococcus aureus. (Health Canada, 2006a). Aims of the present study was to investigate drinking water in wells and tankers, and discover any microbial pathogens in these water as a source of biological environmental health hazard.
Materials and Methods
Sources of water samples
One hundred and eight water samples were collected from different water sources within Makkah city and analysed for bacterial and fungal contamination. Each sample was collected in sterile container sealed with screw cap after disinfection of dispensing point with flame. Then, samples were kept on ice till analysis take place in the laboratory within three hours. There were four sources of water included in this study: governmental sea desalinated water (12 samples), drinkable wells water (36 samples), non-drinkable wells water (36 samples) and small commercial desalination water factories (24 samples).
Sample analysis
Each sample was diluted with sterile distilled water at ratio of 1:10 as final volume 300 ml. Then, each 100 ml from the diluted sample was filtered using filtration equipment system (LabTech, Korea) with fresh cellulose nitrate filter (Sartorius, Germany, with pore size 0.45 μm) for each partition of the diluted sample. The three partitions were poured through filter trap, then two cellulose nitrate filter was taken out carefully by sterile forceps and placed on the MacConkey plate (Biolab, Hungary) and bile esculin plate (Himedia, India) for bacterial growth and the third filter was placed on SD media plate (Himedia, India) for fungal growth. MacConkey and bile esculin plates were incubated for 24 hrs ºC, while SD plate at 25 ºC for 72 hrs (Harley et al., 2002).
Isolation of microorganisms in water samples
Bacterial identification: Colony counter was used for counting of bacterial colonies on cellulose nitrate filters. The used formula for calculating number of bacteria per 100 ml water sample is: No. of bacteria/100 ml = colony dilution x dilution factor. Cellulose nitrate filter was placed and cultured in MacConkey or bile esculine media. Colonies in MacConkey media were pink or yellow color, small size, while colonies in bile esculine media were black in color. On MacConkey media, pink colonies indicate lactose fermented bacteria like E. coli while yellow colonies indicate non lactose fermented bacteria like P. aeroginosa. The non-lactose fermented bacteria were cultured on nutrient agar media to confirm P. aeroginosa that give greenish color colonies. The black colonies in bile esculine media indicate E. faecalis.
a- Gram stain
One drop of saline was mixed with a single colony on slide and fixed with gentle heat. Crystal Violet Oxalate (Atlas, UK) was poured on slide for 2-3 minutes. Gram's Iodine (mordant) was poured on slide for 2-3 minutes. Alcohol decolorized was poured on slide for 1 minute. Safranin counterstain was poured on slide for 2-3 minutes. In each step, the slide was washed with distilled water. The slide was examined microscopically according to Cheesbrough (2007). Gram positive bacteria were blue or violet while gram negative bacteria were pink or red (Momenah, 2004).
b- Brilliant Green Lactose Bile (BGLB) tube test
Every pink colony from MacConkey media was cultured on BGLB (Biolab, Hungary) tube containing inverted Durham's tube. The tube was incubated for 48 hours at 44 C to detect E. coli. The formation of gas in Durham's tube was recorded if the sample was positive (Collee et al., 1989).
c- Indole test
Used to identify E. coli (indole positive). Each pink colonies from MacConkey media was cultured into tube of tryptone water (HIMEDIA, India). The tube incubated at 44 C. A few drops of Kovak's reagent (BioMerieux, France) added the tryptone water culture after the incubation. After gently mixing of tube, positive indole test was indicated by formation of red color in the surface layer within 10 minutes Cheesbrough (2007).
d- Oxidase test
Used to identify P. aeroginosa (oxidase positive). 2-3 drops of freshly prepared oxidase reagent (BDH Laboratories, UK) were added to a piece of filter paper, then the plastic loop was used to take out a colony of the organism that appeared in the culture and placed on the filter paper. In positive cases, blue-purple color appears in few seconds Cheesbrough (2007).
Fungi identification
a- Macroscopic examination
On SD media, texture, surface color and pigment of the reverse (underside) appeared in positive fungal growth. Fungi can cover the whole surface of SD media.
b- Microscopic examination
A small portion of the fungal growth was mixed with drops of Lactophenol Cotton Blue (LPCB)(Bios Europe, UK) on slide. The mixture was tested by using a pair of bent dissecting needles and the slide placed with cover. The cover slip pressed softly by using eraser end of the pencil. The slide tested by low and high power for presence of macroconidia, microcondia, spores and hyphae.
Results
Seven drinkable well water samples (58.3%) and five non-drinkable well water samples (41.7%) were contaminated with E. coli (tables 1,3). Eleven drinkable well water samples (91.7%) and all non-drinkable well water samples (100%) were contaminated with P. aeruginosa (tables 1,4). One drinkable well water samples (8.3%) and 2 non-drinkable well water samples (16.7%) were contaminated with E. faecalis (tables 1,5). Four drinkable water samples (33.3%) and one non-drinkable water sample (8.3%) were found contaminated with aspergillus spp (tables 2,6). Four desalinated water samples (100%) and four private desalinated water samples (50%) were contaminated with Penicillium spp (tables 2,7).
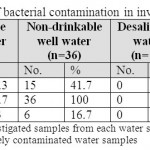 |
Table 1: The percentages of bacterial contamination in investigated water samples Click here to View table |
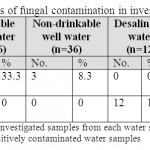 |
Table 2: The percentages of fungal contamination in investigated water samples Click here to View table |
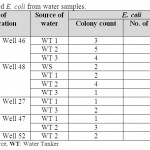 |
Table 3: Isolated E. coli from water samples Click here to View table |
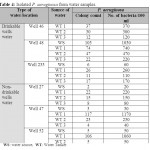 |
Table 4: Isolated P. aeruginosa from water samples Click here to View table |
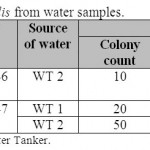 |
Table 5: Isolated E. faecalis from water samples Click here to View table |
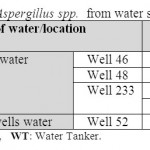 |
Table 6: Isolated Aspergillus spp. from water samples Click here to View table |
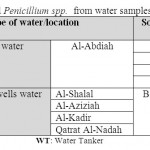 |
Table 7: Isolated Penicillium spp. from water samples Click here to View table |
Discussion
Waterborne pathogens can cause a problem to drinking water supplies, recreational waters, and source waters for agriculture, and aquaculture. Sources of pathogens include municipal wastewater effluents, urban runoff, agricultural wastes and wildlife. A drinking water killed 54 people and induced illness 400,000 in Milwaukee at 1993. Over 200 outbreaks of infectious diseases in Canada associated with drinking water occurred between 1974-1996.
Pathogen contamination of irrigation water or shellfish beds can produce risks to human food supplies. In addition, declines in amphibian populations may be related to fungal or viral pathogens (The National Water Research Institute, Canada, 2001). The microbiological guidelines and standards for drinking water for E. coli, P. aerugnosa and E. faecalis are zero colony count/100 ml of water sample (The Natural Mineral Water, Spring Water and Bottled Drinking Water Regulations, 1999). Periodicity and intensity of rainfall have been shown to impact level of microbial contaminant entering tanks, and the more time between events, the more contaminants accumulate and are washed into the tank (Abbott et al., 2006).
Outbreaks of disease attributable to drinking water in USA still occur and can lead to serious acute, chronic, or sometimes fatal health consequences, particularly in sensitive and immunocompromised populations. From 1971 to 2002, there were 764 waterborne outbreaks associated with drinking water, resulting in 575,457 cases of illness and 79 deaths (Blackburn et al. 2004). Contamination of water is affected by the number of pathogens in the source water, the age of the distribution system, the quality of the delivered water, and climatic conditions. Others have recently estimated waterborne illness rates of 12M cases/year and 16 million cases/yr. (Craun et al. 2006). Reynolds et al., (2008) found that 10.7 million infections and 5.4million illnesses/year occur in populations served by community groundwater systems; 2.2 million infections and 1.1 million illnesses /year occur in noncommunity groundwater systems; and 26.0 million infections and 13.0 million illnesses /year occur in municipal surface water systems.
The total estimated number of waterborne illnesses/yr. in the U.S. is therefore estimated to be 19.5 million/year. The safety of drinking water is evaluated by the results obtained from faecal indicators during the stipulated controls fixed by the legislation. However, drinking-water related illness outbreaks are still occurring worldwide (Figueras and Borrego, 2010). Drinking-water quality in both urban and rural areas of Pakistan is not being managed properly. Most of the drinking-water supplies are faecally contaminated. At places groundwater quality is deteriorating due to the naturally occurring subsoil contaminants or to anthropogenic activities.
The poor bacteriological quality of drinking-water has frequently resulted in high incidence of waterborne diseases while subsoil contaminants have caused other ailments to consumers (Aziz, 2005). In the developing world, an estimated 10 million young children died there in 2006. Of these deaths, WHO estimates that 16.5 percent, or at least 1.65 million, were due to diarrheal diseases, many of which were caused by contaminated water (WHO, 2008). Deaths caused by nondiarrheal infections like typhoid fever are also related to contaminated water (Crump et al., 2040). 1- E.Coli: The obtained results revealed that Escherichia Coli (E. coli) was found in water sources and tankers. Some water tankers were contaminated with E. coli either due to lack of proper treatment and cleaning of tankers or contamination of water well sources. However, the presence of E. coli indicates recent sewage or animal waste contamination (EPA, 2001). E. coli contamination in the present study was found to be higher than that recorded by Golas et al. (2002) which represent 10.7% and Malakauskas et al. (2007) found 16.7% of E. coli contamination in Lithuania different wells.
Admassu et al. (2004) found 28.6% of E. coli contamination in wells water while was 58.33% in 12 water samples (Oyetayo et al., 2007) which is higher than that reported in our study. The survival of enteric bacteria, like Salmonella spp. and E. coli, within the tank environment is influenced by temperature and the presence of nutrients (Leclerc et al., 2002). While leaves are a common source of organic matter, dust, especially during times of drought when particles of ground-based organic and inorganic matter can be carried for hundreds of kilometres during dust storms (Goudie, 2009), is another source. Evans et al. (2007) demonstrated that airborne pathogens from surrounding soils, including E. coli, are significant contributors to the microbial contamination of tanks.
Therefore, in addition to the commonly attributed sources of microbial contaminants, birds, possums or rodents defecating or dying in tanks (Australian Government, 2004), rural participants who graze sheep and cattle, may be exposed to microbial contaminates from higher order-mammals. Exposure to microbial contaminants from higher-order mammals brings with it an increased health risk due to the greater zoonotic potential of such microorganisms.
The Australian Government in its guidance on use of rainwater tanks (Australian Government, 2004) identifies livestock waste as a health hazard only for underground tanks and aerosol waste appears unconsidered. This partitioning of risk, between in-ground and above-ground tanks needs to be reconsidered in light of the work (Evans et al., 2007). Increasing outbreaks (Callaway et al., 2009; Goode et al., 2009) of gastrointestinal illness from livestock-derived E. coli indicates it is perhaps time to rethink the significance of the presence of E. coli in tanks, particularly in rural areas with livestock. In 2006, the most notorious strain of E. coli, STEC O157, was responsible for a waterborne-related outbreak that affected more than 100 people across America and caused at least one death when bacteria transferred from a contaminated water source to a spinach crop that was then packaged and widely distributed (Bettelheim 2007). The survival rate of E. coli in water is varies from 13-245 days (LeJeune et al., 2001). Because it can travel long distances underground, it is can be used as indicator for faecal contamination of ground water (Foppen and Schijven, 2006). In Ireland, bacterial contamination of water is a national concern, with the EPA reporting that over 25% of groundwater samples were contaminated with E. coli in 2004 to 2006. E. coli is the most important indicator used in Ireland and its presence indicates water is unfit for human consumption. It has long been thought that E. coli can only survive for short periods of time in the environment, hence its almost universal use as an indicator of recent faecal contamination of waterways (APA Teagasc, 2010). E. coli O157:H7 was isolated from many water wells in cattle farms. It may be found in water sources, such as private wells, that have been contaminated with feces from infected humans or animals. Waste can enter the water through: sewage overflows, sewage systems that are not working properly, polluted storm water runoff, and agricultural runoff. Wells may be more vulnerable to such contamination after flooding, particularly if the wells are shallow, have been dug or bored, or have been submerged by floodwater for long periods of time (CDC, 2009). The Ministry of Health's Annual Report on Drinking-Water in New Zealand showed unacceptable levels of E. Coli were found in the water of 72,000 or 2 percent of people accessing a registered water supply (Danya, 2011).
Parts of the Danish capital Copenhagen were without clean drinking water after high levels of the E.coli bacteria were detected in the municipal tap water system (Berlingske daily’s Web site, 2011). Australia is particularly vulnerable to threats to both the quality and quantity of drinking water availability because most rainfall evaporates quickly, resulting in twenty percent of the population relying on ground water for drinking supplies which “is extremely difficult to clean up if it becomes polluted” (NHMRC 2004). More than half of the tank water sampled failed to meet the Australian Drinking Water Guidelines for safe drinking water. Levels of E. coli were up to 230x more than the acceptable levels proposed by the Australian Drinking Water Guidelines. Qualitative research found most consumers were unaware of the risks associated with drinking raw rainwater. Further, few took steps to minimize their risk through accepted water management practices (Andrea and Angela, 2010).
Contamination of water samples with Pseudomonas. aeruginosa
Our results showed that Pseudomonas aeruginosa (PA) was the most common microbial contamination in water sources and tankers. All water tankers and majority of water sources (wells) were contaminated due to lack of proper treatment and cleaning. High numbers of PA which represent 1030 bacteria/100 ml were detected in one water source of drinkable well water compared with its water tankers. Also there were high number of PA which represent 1170 bacteria/100 ml in one water tanker of non-drinkable well water. Bari et al. (2007) showed lower PA contamination (4%) from wells than that reported in our study. Geldreich (1996) found that PA was widely distributed in nature and most prevalent opportunistic pathogen isolated from the water samples. P. aeruginosa is part of a large group of free-living bacteria that are ubiquitous in the environment. This organism is often found in natural waters such as lakes and rivers in concentrations of 10/100 mL to >1,000/100 mL.
However, it is not often found in drinking water. Usually it is found in 2% of samples, or less, and at concentrations up to 2,300 mL(-1) or more often at 3-4 CFU/mL. Its occurrence in drinking water is probably related more to its ability to colonize biofilms in plumbing fixtures (i.e., faucets, showerheads, etc.) than its presence in the distribution system or treated drinking water (Mena and Gerba, 2009). Trautmann et al. (2005) in between 1998 and 2005 showed that 9.7% and 68.1% of randomly taken tap water samples on different types of ICUs were positive for PA, and between 14.2% and 50% of infection/colonization episodes in patients were due to genotypes found in ICU water. Although much has been written about biofilms in the drinking water industry, very little has been reported regarding the role of PA in biofilms.
Tap water appears to be a significant route of transmission in hospitals, from colonization of plumbing fixtures (Mena and Gerba, 2009). Outbreaks have been reported from exposure to PA in swimming pools and water slides (Mena and Gerba, 2009). The bacteriological content of water in large dispensers (coolers) and from the 20 liter supply bottles had P. aeruginosa in 25% of samples from the large supply bottles and also in 24% of water specimens from the actual coolers. A further 21.6% of 162 specimens from the coolers yielded P. aeruginosa (Baumgartner and Grand, 2006).
Enterococcus faecalis
In the present study, enterococcus faecalis(E. faecalis) was isolated from drinkable and non-drinkable water samples (8.3% and 16.7%) respectively Ahmed et al., (2005) examined of 12 water samples collected from different wells in Egypt and showed that bacterial contamination Staphylococcus aureus (22.22%), Staphylococcus epidermidis (11.11%), Enterococcus faecalis (11.11%), Bacillus Cereus (55.56%), Yersinia enterocolitica (37.5%), Klebsiella pneumoniae (18.75%), Pseudomonas aeruginosa (12.5%), Escherichia coli (6.25%), Enterobacter agglomerans (6.25%) and Citrobacter freundii (6.25%). These finding were similar to the current results in isolation of P. aeruginosa, E. coli and E. faecalis.
The differences in results may be to several reasons including geographical differences, types of collection method, number of microorganisms which were isolated and type of water samples. Our results revealed that E. faecalis was found in tankers which was the lowest percentage of bacterial contamination. In contrast to our results, Malakauskas et al. (2007) found E. faecalis (23.4%) contamination in may wells. Adbelkarem and Hassan (2000) found that no E. faecalis was detected in wells. In the developing world, 90% of all wastewater still goes untreated into local rivers and streams. About 50 countries, with roughly a third of the world’s population, suffer from medium or high water stress, and 17 of these extract more water annually than is recharged through their natural water cycles. Enterococcus faecalis not only affects surface freshwater sources (rivers and lakes), but it also degrades groundwater resources (UNEP International Environment, 2002).
Failing home septic systems can allow coliforms in the effluent to flow into the water table, aquifers, drainage ditches and nearby surface waters. Sewage connections that are connected to storm drain pipes can also allow human sewage into surface waters. Some older industrial cities in USA use a combined sewer system to handle waste. A combined sewer carries both domestic sewage and storm water. During high rainfall periods, a combined sewer can become overloaded and overflow to a nearby stream or river, bypassing treatment (Walkerton, 2011). Poor quality of water is due to contamination by microorganisms of human or animal origin (Ratajczak et al., 2010). Total coliforms, faecal coliforms, E. coli and enterococci are commonly used microbial indicators of water quality (Davis et al., 2005). Several studies of both recreational and drinking water samples suggested that enterococci are more relevant indicators of faecal contamination than faecal coliforms and E. coli (Grammenouet al., 2006; Kinzelman et al., 2003). Approximately 13% of surface waters in USA do not meet designated use criteria because of high densities of faecal indicator bacteria ("Microbial Source Tracking Guide Document" 2005).
Despite the uncertainty of the effects of animal faecal contamination of ambient waters to human health, microbiological contamination of recreational waters from human faeces is regarded as a greater risk to human health as they are more likely to contain human-specific pathogens. E. coli and Enterococci are considered to have a higher correlation with outbreaks of swimming-associated gastroenteritis than total and faecal coliforms (Wikipedia, 2011). Twelve water sources (9 wells, 3 taps) and 15 latrines were identified and used by 444 inhabitants. Well and tap water showed heavy faecal contamination with more than 1000 CFU/100 ml.
The contamination of drinking water in Bissau due to poor construction, maintenance and improper use ten years after the civil war, demonstrates the need to allocate resources after conflicts in the area of water and sanitation (Colombatti et al., 2009). A total of 300 water samples were collected from 20 different drinking water sources in Kamalapur, Dhaka city from August 2004 to January 2005. The level of faecal contamination was estimated using measurements of faecal indicator bacteria (total coliforms, faecal coliforms and faecal streptococci). The unacceptable level of contamination of total coliforms (TC), faecal coliforms (FC) and faecal streptococci (FS) ranged from 23.8% to 95.2%, 28.6% to 95.2% and 33.3% to 90.0%, respectively (Sirajul Islam et al., 2007). Copeland et al. (2009) measured faecal contamination in 231 randomly primary drinking water samples from selected households. Risk for contamination was compared across source and storage types. A third of samples (30.3%) was contaminated; the source with the highest frequency of contamination was well water (23/24: 95.8%). For tap water, the type of storage had a significant effect on the susceptibility to contamination. The observed pattern of contamination demonstrated the relative potential contributions of both source and storage.
Contamination of water samples with Aspergillus spp.
The recorded results here showed that Aspergillus spp. ontamination in water tankers and water well source. Warris et al. (2001) reported that 21% of drinking water samples have been contaminated with Aspergillus spp. which were higher than that in our obtained results. Aspergillus spp. were recorded by Anaissie et al. (2002) to be more as 70% of all drinking water samples examined. In comparing to this study, our findings were lower contamination. Aspergillus spp. are opportunistic and can cause health problems in immunocompromised patients (Graybill, 2001). In Brazil, 50 people died due to algal toxins in water used for haemodialysis in 1996. Toxins can attack the liver, the nervous system or irritate skin, yet very few of these toxins have been isolated and characterized.
Taste and odour problems in potable water are increasing worldwide and are produced by microorganisms such as bacteria and fungi (The National Water Research Institute, Canada, 2001 ). A total of 197 hot and cold water samples were collected from the main water supply lines and from the taps at three different hospital sites of the University Hospital of Liège.
Filamentous fungi were recovered from 55% and 50% of the main water distribution system and tap water samples, respectively, with a mean of 3.5 and 1.5 colony forming units per 500 ml water. Aspergillus spp. were recovered from 6% of the samples of the water distribution system and A. fumigatus was the most frequently recovered species (66.6%). Fusarium spp. was predominant at one site, where it was found in 28% of tap water samples (Hayette et al., 2010). Two hundred and forty water samples were collected from four university hospitals. 77.5% were positive for fungal growth. Aspergillus (29.7%), Cladosporium (26.7%) and Penicillium (23.9%) were the most common isolated. Among Aspergillus species, A. flavus had the highest frequency. Highest colony counts were found in autumn. Aspergillus predominated in autumn, Cladosporium in winter and spring and Penicillium in summer. This results showed that hospital water should be considered as a potential reservoir of fungi particularly Aspergillus (Hedayati et al., 2011).
Waterborne fungi have been suspected as a source for allergic reaction in sensitive individuals and they may contribute to produce mycotoxins in water (Hageskal et al., 2009). Therefore, study on fungal contamination of water distribution system especially hospitals water has been an interesting subject for many investigators from different countries in the past decade (Hageskal et al., 2006; Kanzler et al., 2008; Pires-Goncalves et al., 2008; Hayette et al., 2010). Gottlich et al. (2002) reported a mean positivity of 26.6% in a Belgian university hospital and groundwater-derived drinking water from water supplies in Germany.
Contamination of water samples with Penicillium spp.
Our results indicated that Penicillium spp. were found in desalinated water and private desalinated water samples with 100% and 50% respectively. Desalinated water tankers supplies water to private desalinated water stations, therefore, Penicillium spp. were detected in both sources. Kanzler et al. (2007) isolated fungi from 38 water wells samples as Cladosporium spp. (74.6%), basidiomycetes (56.4%) and Penicillium spp. (48.7%). This study suggested that drinking water can be a reservoir for opportunistic fungal pathogens.
These pathogens are naturally found in the environment and are not usually regarding as pathogens. However, they can cause disease in human with impaired defence mechanisms like the elderly or young patients with burns, immunosuppressive therapy patient (Hussain et al., 2001). Surface water contamination occurs as a result of direct runoff from waste sites to streams, lakes and wetlands, and indirectly as contaminated groundwater discharges to surface waters. The contamination of groundwater is different from surface water contamination. Because we cannot observe groundwater, we typically discover that the groundwater is contaminated once a well or surface water body becomes contaminated. Surface water contamination occurs quickly and can be stopped at the source. However, groundwater contamination may commence years after the waste source is in place. The slow release rate causes it to take years to thousands of years to move through the groundwater flow regime, and groundwater can be difficult, if not impossible to remediate, and prohibitively costly to remediate. Ultimately all contaminated groundwater will discharge to surface water. Thus, should serious groundwater contamination occur, the destruction of drinking water supplies and aquatic ecosystems occurs for decades to hundreds of years (Coote and Gregorich, 2000).
Effects on human civilization
Water fit for human consumption is called drinking water or potable water. Water is made fit for drinking by filtration, distillation, or by a range of other methods. Poor water quality and bad sanitation are deadly; about five million deaths a year are caused by polluted drinking water. WHO (2010) estimates that safe water could prevent 1.4 million child deaths from diarrhoea each year. The contamination of drinking water sources with microbial pathogens in an on-going problem. More than three million people die every year from water-related disease and 43% of water-related deaths are due to diarrhoea (WHO, 2008). The majority of diseases are infectious in nature caused by bacteria, fungi, viruses and parasites, execrated in human faeces which may lead to contaminate water supplies (Tambekar and Hirulkar, 2007).
Well water is one of the water sources which can be used for population purposes. In the current study, one (16.7%) out of all wells of drinkable well water is clean. Therefore, the remaining wells found to be contaminated with bacterial and fungal pathogens. In Ontario, there are an estimated 500,000 wells which about 10% - 34% of these wells were clean and there were no contamination (Goss et al., 1998). An investigation carried out in Nigeria found that all water samples collected from 15 wells were bacterial contaminated (Olabisi et al., 2008). Same study estimated 20 colony counts per 100 ml as a maximum value, while the present study found that the maximum value of colony counts was 117 per 100 ml. From the obtained results in our study we can conclude that: (1) Certain wells and certain water tankers were found to be contaminated with different microbial pathogens, bacteria and fungi. (2) Penicillium spp. was detected only in desalinated water and private desalinated water before filtration process. (3) The suitable water for drinking is the private desalinated water because they come under different treatment processes like filtration.
Recommendations
Safe water and sanitation pose universal challenges for public health as:
- Periodical monitoring of water sources for pollutants (chemical & microbial).
- Periodical testing of water tankers for their microbial contamination. The risk of microbial contamination in tanks can be reduced by several well-known practices. These include the installation of first flush devices, cleaning gutters, both of which are designed to reduce the build-up of potential contaminants and the use of filtration to remove potential contaminants before use
- Enhanced funding is needed to validate newer molecular detection tools, understand the ecology of pathogens in aquatic ecosystems, better predict disease outbreaks, and improve emergency responses. A preventive approach to pathogen pollution should be taken by developing countries in the form of a source water protection program for all major freshwater sources.
- The identification and control of threats posed by waterborne pathogens will also require effective pathogen detection techniques. The need to develop, evaluate and validate newer molecular tools for pathogen detection such as PCR techniques and DNA microarrays. Rapid advances in fields such as genomics offer the potential to develop improved pathogen detection tools.
- Encourage infrastructure planning, including technological advances, to ensure that improved treatment and environmental protection measures are not diminished by development or population growth.
- Programs are needed to assess the effects of aerial emissions on drinking water quality.
- An improved understanding is needed of methods for assessment and risk analysis of the cumulative effects of agricultural, forestry and other land use activities (e.g., ore, oil and gas exploration) as well as point-source inputs (e.g., municipal and industrial discharges) on surface and ground waters.
References
- Abdelkareem, H. and Hassan, A.A. (2000): Quality assessment of Egyptian drinking water supplies and disinfecting using ultraviolet radiation. Pakistan J. of Biological Sciences, 3(5): 772-776, http://dx.doi.org/10.3923/pjbs.2000.772.776
- Abbott, S.E., J. Ashworth, B.P. Caughley and J. Douwes. 2006. Simple measures for improving the quality of roof-collected rainwater of private dwellings in New Zealand. Proc. of the National small water conf.; Wellington NZ, 3/10/2006.
- Admassu, M.; Wubshet, M. and Gelaw, B. (2004): A survey of bacteriological quality of drinking water in North Gondar. Ethiopian J. of Health Development, 18(2):112-115.
- Ahmed, M.; Aboul-Ela, H.B. and Osman, K.T. (2005): Bacteriological studies on buffaloes farms. Benha Vet. Med. J., 16:2-30.
- Anaissie EJ, Kuchar RT, Rex JH, Francesconi A, Kasai M, Müller FM , et al. (2001): Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: a new paradigm for the epidemiology of opportunistic mold infections. Clin Infect Dis.,33:1871—1878, http://dx.doi.org/10.1086/324501
- Anaissie EJ, Stratton SL, Dignani MC, Lee CK, Summerbell RC, Rex JH, et al. (2002): Pathogenic Aspergillus species recovered from a hospital water system: a 3-year prospective study. Clin Infect Dis., 34:780-789, http://dx.doi.org/10.1086/338958
- Anaissie EJ, Stratton SL, Dignani MC, Lee CK, Summerbell RC, Rex JH, et al. (2003): Pathogenic molds (including Aspergillus species) in hospital water distribution systems: a 3-year prospective study and clinical implications for patients with hematologic malignancies. Blood, 101:2542-2546, http://dx.doi.org/10.1182/blood-2002-02-0530
- Andrea, C. and Angela, T.R. (2010): The E. Coli Load In Self-Managed Rural Water In Australia. The Internet Journal of Microbiology, 9(1).
- APA Teagasc (2010): E. coli as sole indicator of water pollution questioned. ScienceDaily. Retrieved January 1, 2012, from http://www.sciencedaily.com /releases /2010/02 /100226093739.htm
- Australian Government (2004): Guidance on use of rainwater tanks. From http://enhealth.nphp.gov.au.
- Aziz, J.A. (2005): Management of source and drinking-water quality in Pakistan. East Mediterr Health J.,11(5-6):1087-1098.
- Bari, M.D.; Elayse, H.M.; Adalgisa, M.M. and Sato, M.Z. (2007): Aeromonas spp. And microbial indicators in raw drinking water sources. Brazilian J. of Microbiology, 38: 516-521, http://dx.doi.org/10.1590/S1517-83822007000300025
- Bartram J, Lewis K, Lenton R, Wright A. Focusing on improved water and sanitation for health. Lancet. 2005;365(9461):810–812.
- Baumgartner A, Grand M. (2006): Bacteriological quality of drinking water from dispensers (coolers) and possible control measures. J Food Protect., 69:3043-3046.
- Berlingske daily’s Web site (2011): Tap water in Copenhagen contaminated with E.coli. www.homelandsecuritynewswire.com/tap-water-cop..
- Bettelheim, K.A. (2007): The non-O157 shiga-toxigenic (verocytotoxigenic) Escherichia coli; under-rated pathogens. Critic. rev. Micro. 33, 67-87.
- Black PN, Udy AA, Brodie SM. (2000): Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy, 55:501-504, http://dx.doi.org/10.1034/j.1398-9995.2000.00293.x
- Blackburn, B. G., Craun, G. F., Yoder, J. S., Hill, V., Calderon, R. L., Chen, N., Lee, S. H., Levy, D. A. & Beach, M. J. (2004): Surveillance for waterborne-disease outbreaks associated with drinking water—United States, 2001–2002. Morb. Mort. Weekly Report 53(No. SS-8), 23–45.
- Bowyer P, Fraczek M, Denning DW. (2006): Comparative genomics of fungal allergens and epitopes shows widespread distribution of closely related allergen and epitope orthologues. BMC Genomic, 7:251, http://dx.doi.org/10.1186/1471-2164-7-251
- Bramer, S. (2011): "Chemical Nomenclature". Widener University, Dept. of Chemistry. http://science.widener.edu/svb/pset/nomen_b.html.
- Breidenstein EB, de la Fuente-Núñez C, Hancock RE. (2011): Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol.,19:419-26, http://dx.doi.org/10.1016/j.tim.2011.04.005
- Callaway, T.R., M.A. Carr, T.S. Edrington, R.C. Anderson and D. J. Nisbet. (2009): Diet, Escherichia coli O157:H7, and cattle: a review after 10 years. Curr. Issu. Molec. Biol. 11, 67-80.
- CDC (2009): E. coli 0157:H7 and Drinking Water from Private Wells. www. cdc. gov/ healthywater /drinking/.../e_coli.html.
- Cheesbrough, M. (2007): District laboratory practice in tropical countries, 2nd edition. Part 2. Cambridge; New York, Cambridge University Press.
- Coote, D.R. and L.J. Gregorich. (2000): The health of our water--towards sustainable agriculture in Canada. Research Planning and Coordination Directorate, Research Branch, Agriculture and Agri-Food Canada, Ottawa, Canada. 173 p.
- Collee, J.G.; Duguid, J.P.; Fraser, A.G. and Marmion, B.P. (1989): Mackie and McCartney Practical Medical Microbiology, 13th edition. Churchill Livingstone, pp. 793-812.
- Colombatti, R.; C S Vieira, F Bassani, R Cristofoli, A Coin, L Bertinato, F Riccardi (2009): Contamination of drinking water sources during the rainy season in an urban post-conflict community in Guinea Bissau: implications for sanitation priority. African journal of medicine and medical sciences, 38 (2): 155-161.
- Copeland, C.C.; Beers, B.B.; Thompson, M.R.; Fitzgerald, R.P. and Barrett, L.J. (2009): Faecal contamination of drinking water in a Brazilian shanty town: importance of household storage and new human faecal marker testing. Journal of Water and Health, 7(2): 324-331, http://dx.doi.org/10.2166/wh.2009.081
- Craun, M.F.; Craun, G.F.; Calderon, L.R. and Beach, M.J. (2006): Waterborne outbreaks reported in the United States. J. of Water and Health, 4(2):19-30, http://dx.doi.org/10.2166/wh.2006.016
- Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bulletin of the World Health Organization. 2004;82(5):346–353.
- Danya, L. (2011): Unacceptable" levels of E.Coli in drinking water. Fairfax New Zealand Limited Privacy www.stuff.co.nz/.../Unacceptable-levels-of-E-Coli-in...A new report shows 72,000 of New Zealanders are drinking faecally-contaminated water.
- Davis K, Anderson MA, Yates MV: Distribution of indicator bacteria in Canyon Lake, California. Water Res 2005, 39(7):1277-1288, http://dx.doi.org/10.1016/j.watres.2005.01.011
- Doyle, M. P., and M. C. Erickson. 2006. "Closing the door on the faecal coliform assay." Microbe 1:162-163. ISSN 1558-7460.
- EHA (Environmental & Public Health Consulting Group) (2012): What is pseudomonas aeruginosa? www.ehagroup.com/.
- EPA (2001): preparing your drinking water consumer confidence report: Revised guidance for water suppliers. Retrieved November 26, 2008 from http://www.epa.gov/OGWDW/ccr/pdfs/guide_ccr_forwatersuppliers.pdf
- EPA (2011): Basic Information about Pathogens and Indicators in Drinking Water. water.epa.gov/drink/contaminants/.../pathogens.cfm.
- Evans, C.A., P.J. Coombes, R.H. Dunstan, and T. Harrison. (2007): Identifying the major influences on the microbial composition of roof harvested rainwater and the implications for water quality. Water Sci. Tech. 55(4), 245-253, http://dx.doi.org/10.2166/wst.2007.115
- Figueras MJ, Borrego JJ. (2010): New perspectives in monitoring drinking water microbial quality. Int J Environ Res Public Health, 7(12):4179-202, http://dx.doi.org/10.3390/ijerph7124179
- Foppen, J.W.A. and Schijven, J.F. (2006): Evaluation of data from the literature on the transport and survival of E. coli and thermotolerant coliforms in aquifers under saturated conditions. Water Research, 40(3): 401-426, http://dx.doi.org/10.1016/j.watres.2005.11.018
- Geldreich, E.E. (1996): Characterizing microbial quality of water supply: In microbial quality of water supply in distribution systems, 1st edition. CRC Incorporation, Boca Raton, Florida, USA: 236.
- Golas, I.; Filipkowska, Z.; Lewandowska, D. and Zmyslowska, I. (2002): Potentially pathogenic bacteria from the family Enterobacteriaceae, Pseudomonas spp. and Aeromonas spp. In water designated for drinking and household purposes. Polish J. of Environmental Studies, 11(4):325-330.
- Goode, B., C. O’Reilly, J. Dunn, K. Fullerton, S. Smith, G. Ghneim, J. Keen, L. Durso, M. Davis and S. Montgomery. 2009. Outbreak of Escherichia coli O157H7 infections after petting zoo visits, North Carolina State Fair, October-November 2004. Arch. Ped. Adol. Med. 163 (1), 42-48, http://dx.doi.org/10.1001/archpediatrics.2008.525
- Gottlich E, Van der Lubbe W, Lange B, Fiedler S, Melchert I, Reifenrath M, et al. (2002): Fungal flora in groundwater-derived public drinking water. Int J Hyg Environ Health, 205:269-279, http://dx.doi.org/10.1078/1438-4639-00158
- Goss, M.J., Barry, D.A.J. and Rudolph, D.L. (1998): Contamination of Ontario farmstead domestic wells and its association with agriculture: Results from drinking water wells. J. of Contaminant Hydrology, 32:267-293, http://dx.doi.org/10.1016/S0169-7722(98)00054-0
- Goudie, A.S. (2009): Dust storms: recent development. J. Enviro. Manag. 90, 89-94, http://dx.doi.org/10.1016/j.jenvman.2008.07.007
- Grammenou P, Spiliopolullou I, Sazakli E, Papapetropoulou M (2006): PFGE analysis of enterococci isolates from recreational and drinking water in Greece. J Water Health 2006, 4(2):263-269.
- Graybill, J.R. (2001): The echinocandins, first novel class of antifungals in two decades: Will they live upto their promise? International J. of Clinical Practice, 55(9):633-638.
- Hageskal G, Knutsen AK, Gaustad P, de Hoog GS, Skaar I. (2006): Diversity and significance of mold species in Norwegian drinking water. Appl Environ Microbiol., 72:7586—93, http://dx.doi.org/10.1128/AEM.01628-06
- Hageskal G, Lima N, Skaar I. (2009): The study of fungi in drinking water. Mycol Res., 113:165-172, http://dx.doi.org/10.1016/j.mycres.2008.10.002
- Harley, J.P.; Prescott, L.M. and Klein, D.A. (2002): Laboratory excercises in microbiology, 5th edition. Boston, McGraw-Hill.
- Hayette, M-p.; Christiaens, G.; Mutsers, J.; Barbier, C.; Huynen, P.; Melin, P. and De Mol, P. (2010): Filamentous fungi recovered from the water distribution system of a Belgian university hospital. Medical mycology official publication of the International Society for Human and Animal Mycology, 48 (7): 969-974.
- Health Canada (2008): Drinking Water Contaminants - Escherichia coli, E. coli. www.freedrinkingwater.com/water.../ecoli-bacteria-r.
- Health Canada (2006a) Guidelines for Canadian drinking water quality: Guideline Technical Document--Bacterial waterborne pathogens: Current and emerging organisms of concern. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
- Hedayati, M.T.; S. Mayahi, M. Movahedi, T. Shokohi (2011): Study on fungal flora of tap water as a potential reservoir of fungi in hospitals in Sari city, Iran. Journal de Mycologie Médicale, 21, 10-14, http://dx.doi.org/10.1016/j.mycmed.2010.12.001
- Hedayati M.T., Pasquallotto AC, Warn PA, Bowyer P, Denning DW. (2007): Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology, 153:1677—92, http://dx.doi.org/10.1099/mic.0.2007/007641-0
- Hussain, I.; Awan, S.; Hussain, T. and Sarwar, Z. (2001): Microbia study of drinking water from Rawalakot and its surroundings. J. of Biological Sciences, 1(4): 287-288, http://dx.doi.org/10.3923/jbs.2001.287.288
- Jolanta, S. (2005): Evaluation of mycological contamination of dental unit waterlines. Annals of Agricultural and Environmental Medicine, 12:153-155.
- Kanzler, D.; Buzin, W.; Paulitsch, A.; Hass, D.; Platzer, S.; Marth, E. and Mascher, D. (2007): Occurrence and hygienic relevance of fungi in drinking water. Mycoses, 51(2):165-169,http://dx.doi.org/10.1111/j.1439-0507.2007.01454.x
- Kanzler D, Buzina W, Paulitsch A, Haas D, Platzer S, Marth E, et al. (2008): Occurrence and hygienic relevance of fungi in drinking water. Mycoses, 51:165-169, http://dx.doi.org/10.1111/j.1439-0507.2007.01454.x
- Kinzelman J, Ng C, Jackson E, Gradus S, Bagley R (2003): Enterococci as Indicators of Lake Michigan Recreational Water Quality: Comparison of Two Methodologies and Their Impacts on Public Health Regulatory Events. Appl Environ Microbiol, 69(1):92-96, http://dx.doi.org/10.1128/AEM.69.1.92-96.2003
- LeJeune, J.T.; Besser, T.E. and Hancock, D.D. (2001): Cattle water troughs as reservoirs of E. coli O157. Applied and Environmental Microbiology, 67:3053-3057, http://dx.doi.org/10.1128/AEM.67.7.3053-3057.2001
- Leclerc, H., L. Schwartzbrod and E. Del-Cas. (2002): Microbial agents associated with waterborne diseases. Crit. Rev. Micro. 28(4), 371-409, http://dx.doi.org/10.1080/1040-840291046768
- Malakauskas, M.; Kasnauskyte, N.; Kudirkiene, E.; Serniene L.; Malakauskas, A.; Stimbirys, A. and Milius, J. (2007): Microbial evaluation of drinking water from centralized and small community supply systems in Kaunas region, Lithuania, 1st edition. Lithuania Vet. Academy, Department of Food Safety and Animal Hygiene.
- MDG Report (2008): http://mdgs.un.org/unsd/mdg/Resources/Static/Products/Progress2008/MDG_Report_2008_En.pdf#page=44. Retrieved 2010-07-25.
- Mena KD, and Gerba CP (2009): Risk assessment of pseudomonas aeruginosa in water. Rev Environ Contam Toxicol. 2009;201:71-115, http://dx.doi.org/10.1007/978-1-4419-0032-6_3
- "Microbial Source Tracking Guide Document." Environmental Protection Agency. June (2005). Retrieved on 4 September 2010 from http: //www. epa.gov/nrmrl/pubs/600r05064/600r05064.pdf
- Momenah, A. (2004): Concepts in medical microbiology,1st edition.Umm Al-Qura University.
- NHMRC (National Health and Medical Research Council) (2004): Australian Drinking Water Guidelines. NHMRC, Sydney NSW.
- Olabisi, O.E.; Awonusi, A.J. and Adebayo, O.J. (2008): Assessment of bacterial pollution of shallow well water in Abeokuta, Southwestern Nigeria. Life Science J., 5:59-65.
- Oyetayo, V.O.; Akharaiyi, F.C. and Oghumah, M. (2007): Antibiotic sensitivity pattern of Escherichia coli isolated from water obtained from wells in Akure Metropolis. Research J. of Microbiology, 2(2):190-193.
- Pires-Gonçalves RH, Sartori FG, Montanari LB, Zaia JE, Melhem MS, Mendes-Giannini MJ, et al. (2008): Occurrence of fungi in water used at a haemodialysis centre. Lett Appl Microbiol., 46:542-547, http://dx.doi.org/10.1111/j.1472-765X.2008.02349.x
- Pitt, J.L.; Samson, R.A.; and Frisvad, J.C. (2000): List of accepted species and their synonyms in the family Trichocomaceae. In: Integration of modern methods for Penicillium and Aspergillus classification. Samson, R.A. & Pitt, J.L.(editors). pp.9-49.
- Pollack, G. (2011): "Water Science". University of Washington, Pollack Laboratory. http://faculty.washington.edu/ghp/researcthemes/water-science.
- Prüss-Üstün A, Bos R, Gore F, Bartram J.Safer water, better health: costs, benefits and sustainability of interventions to protect and promote health. Geneva: World Health Organization; 2008.
- Rajendran, P.; Murugan, S.; Raju, S.; Sundaraj, T.; Kanthesh, B.M. and Reddy, E.V. (2006): Bacteriological analysis of water samples from from Tsunami hit coastal areas of Kanyakumari District, Tamil Nadu. Indian J. of Med. Microbiology, 24:114-116, http://dx.doi.org/10.4103/0255-0857.25188
- Ratajczak M, Laroche E, Berthe T, Clermont O, Pawlak B, Denamur E, Petit F (2010): Influence of hydrological conditions on the Escherichia coli population structure in the water of a creek on a rural watershed. BMC Microbiol, 10:
- Reynolds KA, Mena KD, Gerba CP. (2008): Risk of waterborne illness via drinking water in the United States. Rev Environ Contam Toxicol.;192:117-158.
- Sirajul Islam, M.; A Brooks, M S Kabir, I K Jahid, M Shafiqul Islam (2007): Faecal contamination of drinking water sources of Dhaka city during the 2004 flood in Bangladesh and use of disinfectants for water treatment. Journal of Applied Microbiology, 103(1): 80-87, http://dx.doi.org/10.1111/j.1365-2672.2006.03234.x
- Sammon NB, Harrower KM, Fabbro LD, Reed RH. (2011): Three potential sources of microfungi in a treated municipal water supply system in sub-tropical Australia. Int J Environ Res Public Health, 8(3):713-732, http://dx.doi.org/10.3390/ijerph8030713
- Sammon NB, Harrower KM, Fabbro LD, Reed RH.(2010): Incidence and distribution of microfungi in a treated municipal water supply system in sub-tropical Australia. Int J Environ Res Public Health, 7(4):1597-611, http://dx.doi.org/10.3390/ijerph7041597
- Simon-Nobbe B, Denk U, Schneider PB, Radauer C, Teige M, Crameri R, et al. (2006): NADP-dependent mannitol dehydrogenase, a major allergen of Cladosporium herbarum. J Biol Chem., 281:16354-16360, http://dx.doi.org/10.1074/jbc.M513638200
- Sanchez-Carrillo C, Padilla B, Marín M, Rivera M, Cercenado E, Vigil D, Sanchez-Luna M, Bouza E (2009): Contaminated feeding bottles: the source of an outbreak of Pseudomonas aeruginosa infections in a neonatal intensive care unit. Am J. Infect. Control, 37:150-154, http://dx.doi.org/10.1016/j.ajic.2008.04.259
- Tambekar, D.H. and Hirulkar, N.B. (2007): Rapid and modified field test for detection of faecal contamination in drinking water. J. of Scientific and Industrial Research, 66(4):667-669.
- The Natural Mineral Water, Spring Water and Bottled Drinking Water Regulations (1999): Statutary Instrument No. 1540. London: Stationary Office.
- The National Water Research Institute, Canada (2001): Workshop in Toronto about: Threats to Sources of Drinking Water and Aquatic Ecosystem Health in Canada.
- Todar, K. (2012): Todar's Online Textbook of Bacteriology. www. Textbook of bacteriology .net
- Trautmann, M.; MD, Philipp M. Lepper, P.M.; and MD, Mathias Haller, M. (2005): Ecology of Pseudomonas aeruginosa in the intensive care unit and the evolving role of water outlets as a reservoir of the organism. American Journal of Infection Control, 33 (5): S41–S49, http://dx.doi.org/10.1016/j.ajic.2005.03.006
- U.S. EPA (Environmental Protection Agency) (2000): Water quality conditions in the United States. A profile from the 1998 National Water Quality Inventory Report to Congress. Report EPA 841-F-00-006, U.S. EPA, Office of Water, Washington, DC.
- UNEP International Environment (2002). Environmentally Sound Technology for Wastewater and Stormwater Management: An International Source Book. IWA Publishing. ISBN 1843390086. OCLC 49204666.
- Walkerton, T. (2011): Escherichia coli. From Wikipedia, the free encyclopedia, en. wikipedia. org/wiki/Escherichia_coli.
- Warris A, Gaustad P, Meis JF, Voss A, Verweij PE, brahamsen TG. (2001): Recovery of filamentous fungi from water in a paediatric bone marrow transplantation unit. J Hosp Infect., 47: 143-148, http://dx.doi.org/10.1053/jhin.2000.0876
- WHO (2008): Safer water, Better Health: Costs, benefits and sustainability of interventions to protect and promote health. Retrieved January 10, 2009 from http://whqlibdoc.who.int/publications/2008/9789241596435_eng.pdf
- WHO/UNICEF. Meeting the MDG water and sanitation target: the urban and rural challenge of the decade. New York and Geneva: UNICEF and WHO; 2006.
- Wikipedia, the free encyclopedia (2011): E. coli O104:H4 outbreak (Redirected from 2011 E. coli O104:H4 outbreak) www.amnh.org/nationalcenter/.../2011/joshua.html.







