Mercury Induced Biochemical Alterations As Oxidative Stress In Mugil Cephalus In Short Term Toxicity Test
J.S.I Rajkumar1 * and Samuel Tennyson2
DOI: http://dx.doi.org/10.12944/CWE.8.1.06
Copy the following to cite this article:
Rajkumar J. S. I, Tennyson s. Mercury Induced Biochemical Alterations As Oxidative Stress In Mugil Cephalus In Short Term Toxicity Test. Curr World Environ 2013;8(1) DOI:http://dx.doi.org/10.12944/CWE.8.1.06
Copy the following to cite this URL:
Rajkumar J. S. I, Tennyson S. Mercury Induced Biochemical Alterations As Oxidative Stress In Mugil Cephalus In Short Term Toxicity Test. Curr World Environ 2013;8(1). Available from: http://www.cwejournal.org/?p=3066
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-02-11 |
|---|---|
| Accepted: | 2013-03-21 |
Estuarine pollution is an ongoing activity started long back however intensified during the last few decades, and currently the circumstances has become alarming, especially in India1. Metals are natural components; however become contaminants of the aquatic environment, due to anthropogenic activities2. Bioavailability and indestructible nature are the most fundamental property of heavy metal exerting toxic effects on living organisms when they exceed a certain concentration limit3.
Heavy metals in metal accumulating organisms are linked to their ability to bind incoming metals, thereby controlling their intracellular availability leading to tolerance ability of test organisms. Oxidative stress induced by metals could be the best indicator and often interpreted as a failure of detoxification mechanisms in metal active sites such as mitochondria4. Cellular measurements and its responses to chemical contaminants like heavy metals in test organisms are used as bio-indicators from aquatic environment allowing early detection of biological effects as well as assessment of the extent of contamination of pollutants 5, 6. Depletion of glutathione and sulfhydryl groups of protein due to heavy metals results in increased Reactive oxygen species (ROS) production such as, hydrogen peroxide, superoxide anion and hydroxyl radicals7.
Superoxide anion and hydrogen peroxide is generated from sequential reduction of oxygen8. Another reactive species peroxynitrite is produced when superoxide anion rapidly reacts with nitric oxide and has the potential to trigger cellular death9. ROS are measured as crucial intermediaries for the metal-triggered tissue injuries and apoptosis7. There must be effective anti-oxidation systems in the organisms to prevent oxidation induced damage. Some components of anti-oxidation systems involve reduced glutathione (GSH) and antioxidant enzymes including free radical scavenging enzymes, such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidases (GPX) and Glutathione Reductase (GR). Other related enzymes are Glyoxalase I (GI), Glyoxalase II (GII) and Glutathione S-Transferase (GST). GSH reduces ROS under oxidative stress, with the concomitant formation of the oxidized glutathione (GSSG) 10. Particularly in the aquatic environment, oxidative stress is one of the ecological significance, providing a sink for many pollutants that are capable of causing oxidative stress 11. Alterations in the activity of enzymes and related biomarkers are the potential tools for aquatic toxicological research12. Fish being a source in nutrient cycling and maintaining community balances in aquatic ecosystem. They play an important role in energy flow and are regarded as high protein to man13. Hence convenience of fish for assessing environmental conditions in aquatic ecosystem as test organisms has gained eminence in recent years14. Fish are considered as suitable bio-monitors for environmental pollution and they are exposed to the heavy metals in vitro and to study the effects of heavy metals in aquatic ecosystems15.
The study related to antioxidant defense system is being increasingly reported due to its potential ability to provide biochemical biomarkers that can be used in environmental monitoring system such as aquatic pollution and estuarine contamination in specific11. Tools involving biomarkers in environmental monitoring confer significant advantages over traditional chemical measurements because measured biological effects can be meaningfully linked to environmental consequences so that environmental concerns can be directly addressed16. Hence, in the present study the biochemical parameters such as lipid peroxidation levels, Glutathione S-transferase, catalase, reduced glutathione and acetylcholinesterase were measured by exposing juveniles of Mugil cephalus to mercury under short-term toxicity tests (static renewal).
MATERIAL AND METHODS:
Fingerlings of Mugil cephalus of mean 2.5 ±0.6cm in length and 0.13 ±0.02g in weight were selected for the study. Collected juveniles were immediately transported to the laboratory in air filled plastic bags and acclimatized fish fingerlings in 200 L Fiberglass Reinforced Plastics (FRP) tanks with aerated natural filtered seawater. Stock solutions of mercury were freshly prepared by dissolving mercury (II) chloride in de-ionized (double distilled) water. Fresh stock solutions were prepared daily. These solutions were serially diluted to get the experimental concentration for the toxicity test. The experimental method includes static renewal (24 hour renewal) test 17. Five concentrations in a geometric series including control were prepared for the test for 14 days in short-term chronic toxicity test 18. Toxicant and seawater were replaced on daily basis. Test animals were fed three times during the test. Maximum-allowable control mortality was 20 per cent for short-term chronic toxicity test18. At the final stages of the toxicity test, the tissue samples of survived test animals were pooled and made in duplicates. For the analysis of lipid peroxidation marker and antioxidant enzyme activities, 1g tissue was homogenized in chilled pestle and mortar with 5ml homogenization buffer (0.25Msucrose, 10 mMTris, 1 mMEDTA, and pH 7.4) and centrifuged at 5,000 rpm for 15 minutes at 4°C. The resulting supernatant was the homogenate which was used for the estimation of various biochemical assays.
LIPID PEROXIDATION (LPO
Lipid peroxidation level was assayed by measuring Malondialdehyde (MDA), a decomposed product of polyunsaturated fatty acids. Hydro peroxides were determined by the thio-barbituric acid reaction and was measured at 532 nm in the UV-Spectrophotometer19. The amount of Thio-barbituric Acid Reactive Substance (TBARS) was calculated by using an extinction coefficient of 1.56 x 105/M/cm and expressed as nmol TBARS formed /mg protein.
GLUTATHIONE S-TRANSFERASE (GST)
Activity of Glutathione S-transferase (GST) was assayed at 340 nm by measuring the increase in absorbance using 1-chloro-2, 4-dinitrobenzene (CDNB) as the substrate20. The results were expressed as nM of GSH and CDNB conjugate formed /min/mg protein. Values expressed as nanomoles of reduced glutathione and CDNB conjugate formed/min/mg protein.
CATALASE (CAT)
Catalase (CAT) activity was measured at 240 nm by determining the decay of hydrogen peroxide levels and was expressed as µmol of hydrogen peroxide consumed /min/mg/protein21.
REDUCED GLUTATHIONE (GSH)
The reduced glutathione (GSH) was measured at 412 nm using 5, 5-dithiobis-(2-nitrobenzoic acid) (DTNB) reagent 22. The values were expressed as µmol of GSH oxidized/mg protein.
ACETYLCHOLINESTERASE ACTIVITY (AChE)
Acetylcholine esterase activity (AChE) activity was determined using Ellman’s reagent, DTNB (5, 5’-dithio-bis (2- nitrobenzoic acid); 0.5mM) and acetylthiocholine iodide (ACTI) as substrate 23, 24, 25. The rate of change of absorbance at 412 nm was recorded over 1.5 minutes at 25°C. The protein concentration of each of the sample extract was determined measured at 750 nm in UV-Spectrophotometer26. One-way ANOVA (Dunnetts procedure) was used to compare the results with control using graphpad prism version 5.
RESULTS AND DISCUSSION '
Scavenging enzymes at lower concentration in juvenile fish makes them vulnerable to oxidative damage when attacked by ROS 27. M.cephalus exposed to exposure concentrations experienced rigorous Oxidative stress (OS) characterized by significant alterations in biomarkers, were also been observed in brain samples of the mullet28. Removal of H2O2 is an important strategy of marine organisms against oxidative stress29. Increased activities of CAT have been reported in several fish and invertebrate species 30, 31. Concentration of LPO was significantly higher (P< 0.001) in higher concentrations of mercury due to increased levels of exposure indicating the importance of antioxidant32.
The level of total protein to mercury exposure significantly (P<0.001) decreased in 10 µg/l. Glutathione-S-transferase (GST) exhibited a significant (P<0.001) increase in the activity at 8 and 10 µg/l concentration of mercury. Reduced glutathione (GSH) level significantly (P<0.001and 0.05) decreased in the 14 days of exposure compared to control in all the concentrations. Catalase (CAT) and lipid per oxidation (LPO) showed trend of significant decrease and increase in linear increase in the mercury concentration. The activity of acetylcholinesterase (AChE) significantly (P<0.01 and 0.05) decreased throughout the exposure concentration. M.cephalus exposed to lead in short-term chronic toxicity test showed that all the biochemical components and anti-oxidative enzymes of the oxidative stress showed significant changes in the tissues exposed concentration of mercury. The results are represented as Table 1.
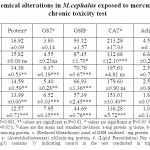 |
Table 1. Biochemical alterations in M.cephalus exposed to mercury in short-term chronic toxicity test Click here to View table |
Protein content in M.cephalus might be due to the proteolysis process for energy production and utilization owing to the decreased food intake of test organisms under stress33. These data may indicate a faster rate of GSH utilization or degradation, which could be responsible for the observed lower GSH content. Moreover, increase of GSH content may be related to prevention of oxidative challenge 34. Aquatic organisms maintain high content of GSH in tissues and increased content has the function of protection that could provide the first line of defense against the influence of toxic heavy metals 35, 36. Esterases are considered as potential biomarkers to differentiate the levels of contaminants37.
Maintenance of enzyme activities in relation to oxidative stress may serve as important markers of the presence of hazardous substances38, 39. Mullet (Mugil sp.) from contaminated Spanish areas revealed increased activities of antioxidant (catalase) and detoxifying Glutathione S-Transferase (GST) enzymes 40, 41. Channel catfish (Ictalurus punctatus) exposed to effluents resulted in a significant increase in catalase activity42. Changes in GST activity exhibit detoxification process in fish exposed to toxic compounds4, 5. Decrease of GST was observed activity in fish exposed to mercury in the present study. This induction in GST activity could indicate a defense of fish against oxidative stress damage produced by adverse conditions such as heavy metal contamination. Increased levels of lipid peroxidation (LPO) have been observed in fish under experimental conditions, upon exposure to different xenobiotics43.
There are evidences that heavy metals like those used in the studied, produced increased LPO levels in M.cephalus44. The concurrent use of several biomarkers is important to minimize misinterpretation in cases of complex situations of pollution45. The result indicates that fish actively generate oxidative stress and antioxidant responses which can be used as biomarkers of pollution.
REFERENCES:
- Girija T.R., Mahanta C. and Chandramouli V., Environ. Monitor. Assess., 130: 221-236, (2007), http://dx.doi.org/10.1007/s10661-006-9391-6
- MacFarlane G.R. and Burchett M.D., Aquat. Bot., 68: 45-59, (2000), http://dx.doi.org/10.1016/S0304-3770(00)00105-4
- Ridgway J. and Shimmield G., Estua. Coas. Shelf Sci., 55: 903-928, (2002), http://dx.doi.org/10.1006/ecss.2002.1035
- Ballesteros M.L., Wunderlin D.A. and Bistoni M.A., Ecotoxicol. Environ. Saf., 72: 199-205, (2009a), http://dx.doi.org/10.1016/j.ecoenv.2008.01.008
- Ballesteros M.L., Durando P.E., Nores M.L., Diaz M.P., Bistoni M.A. and Wunderlin D.A., Environ. Poll., 157: 1573-1580, (2009b), http://dx.doi.org/10.1016/j.envpol.2009.01.001
- Scott G.R. and Sloman K.A., Aquat. Toxicol., 68: 369-392, (2004), http://dx.doi.org/10.1016/j.aquatox.2004.03.016
- Liu J., Qu W. and Kadiiska M.B., Toxicol. Appl. Pharmacol., 238: 209-214, (2004), http://dx.doi.org/10.1016/j.taap.2009.01.029
- Monferran M.V., Pesce S.F., Cazenave J. and Wunderlin D.A., Environ. Toxicol., 23: 184-192, (2008), http://dx.doi.org/10.1002/tox.20326
- Vieira L.R., Gravato C., Soares A.M.V.M., Morgado F. and Guilhermino L., Chemos., 76: 1416-1427, (2009), http://dx.doi.org/10.1016/j.chemosphere.2009.06.005
- Lushchak V.I. and Bagnyukova T.V., Comp. Biochem. Physiol. Part B Biochem. Mol. Biol., 148(4): 390-397, (2007).
- Oliveira M., Ahmad I., Maria V.L., Pacheco M. and Santos M.A., Ecotoxicol., 19: 643-653, (2010), http://dx.doi.org/10.1007/s10646-009-0436-9
- Arellano J.M., Blasco J., Ortiz J.B., Da’Silva D.C., Navarro A., Del-Pino M.J.S. and Sarasquete C., Ecotoxicol. Environ. Restor., 3(1): 22-28, (2000).
- Tuzen M., Food Chem., 80: 119-123, (2003), http://dx.doi.org/10.1016/S0308-8146(02)00264-9
- Sikorska J. and Wolnicki J., Rev. Fish. Biol. Fisheries., 20: 417-423, (2010), http://dx.doi.org/10.1007/s11160-009-9145-y
- Padmini E., Hepshibha B.T. and Shellomith, A.S.S., Aquacult., 5(1): 115-118, (2004).
- Wu R.S.S., Siu W.H.L. and Shin P.K.S., Mar. Poll. Bull., 51: 623-634, (2005), http://dx.doi.org/10.1016/j.marpolbul.2005.04.016
- United States Environmental Protection Agency, 2, Washington, DC, USA, (2002a).
- United States Environmental Protection Agency, EPA 833- R-00-003, (2002b).
- Ohkawa H., Anal. Biochem., 95: 351-358, (1979), http://dx.doi.org/10.1016/0003-2697(79)90738-3
- Habig, W.H., Papst M.J. and Jacoby W.B., J. Biol. Chem., 249: 7130-7139, (1974).
- Beers R.F. and Seizer I.W., J. Biol. Chem., 115: 133-140, (1952).
- Moron M.S., Beperre J.W. and Wick B., Biochem. Biophys. Acta., 582: 67-78, (1979), http://dx.doi.org/10.1016/0304-4165(79)90289-7
- Ellman G.L., Courtney K.D., Andres V. and Featherstone R.M., Biochem. Pharmacol., 7: 88-95, (1961), http://dx.doi.org/10.1016/0006-2952(61)90145-9
- Najimi S., Bouhaimi A., Daubeze M., Zekhnini A., Pellerin J., Narbone J.F. and Moukri A., Bull. Environ. Cont. Toxicol., 58: 901-908, (1979), http://dx.doi.org/10.1007/s001289900419
- Alves S.R., Severino C., Ibbotson P.C., Silva D.P., Lopes A.Z., Saenz F.R.A.S. and Bainy L.A., Mar. Environ. Res., 54: 241-245.
- Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.J., J. Biol. Chem., 193: 265-275, (1951).
- Runnalls T.J., Hala D.N. and Sumpter J.P., Aquat. Toxicol., 84: 111-118, (2007), http://dx.doi.org/10.1016/j.aquatox.2007.06.005
- Padmini E. and Kavitha M., Poll. Res., 24(3): 505- 508, (2005).
- Valavanidis A., Vlahogianni T., Dassenakis M. and Scoullos M., Ecotoxicol. Environ. Saf., 64: 178-189, (2006), http://dx.doi.org/10.1016/j.ecoenv.2005.03.013
- Stephensen E., Svavarsson J., Sturve J., Ericson G., Erici M.A. and Forlin L., Aquat. Toxicol., 48: 431-442, (2000), http://dx.doi.org/10.1016/S0166-445X(99)00062-4
- Regoli F. and Principato G., Aquat. Toxicol., 31: 143-164, (2005), http://dx.doi.org/10.1016/0166-445X(94)00064-W
- Pampanin D.M., Camus L., Gomiero A., Marangon I., Volpato E. and Nasci C., Mar. Poll. Bull., 50: 1548-1557, (2005), http://dx.doi.org/10.1016/j.marpolbul.2005.06.023
- Elumalai M. and Balasubramanian M.P., Bull. Environ. Contam. Toxicol., 62(6): 743-748, (2009), http://dx.doi.org/10.1007/s001289900935
- Dandapat J., Chainy G.B.N. and Rao J.K., Comp. Biochem. Physiol. Part C, 127: 101-115, (2000).
- Thomas P. and Juedes M.J., Aqua. Toxicol., 23: 11-30, (1992), http://dx.doi.org/10.1016/0166-445X(92)90009-C
- Son M.H., Kang K.W., Lee C.H. and Kim S.G., Biochem. Pharmacol., 62: 1379-1390, (2001), http://dx.doi.org/10.1016/S0006-2952(01)00780-8
- Konradt J. and Braunbeck T., J. Aquat. Ecosys. Stress Recov., 8: 299-318, (2001), http://dx.doi.org/10.1023/A:1012928914322
- Oost V.R., Beyer J. and Vermeulen N.P.E., Environ. Toxicol. Pharmacol., 13(2): 57-149, (2003), http://dx.doi.org/10.1016/S1382-6689(02)00126-6
- Braunbeck T., Fish Ecotoxicolgy, Experientia., Suppl. Ser., 61-140, (1998).
- Livingstone D.R., Mar. Poll. Bull., 42(8): 656-666, (2001), http://dx.doi.org/10.1016/S0025-326X(01)00060-1
- Ariza R.A., Mendez F.M.D., Navas J.I., Pueyo C. and Barea J.L., Environ. Mol. Mutag., 25: 50-57, (1995), http://dx.doi.org/10.1002/em.2850250108
- Mihaich M.E. and Di-Giulio R.T., Arch., Environ. Contam. Toxicol., 20: 391-397, (1991), http://dx.doi.org/10.1007/BF01064409
- Bhattacharya S., Bhattacharya A. and Roy S., Fish Physiol. Biochem., 33: 463-473, (2007), http://dx.doi.org/10.1007/s10695-007-9173-2
- Talas Z.S., Orun I., Ozdemir I., Erdogan K., Aldan A. and Yilmaz I., Fish Physiol. Biochem., 34: 217-222, (2008), http://dx.doi.org/10.1007/s10695-007-9179-9
- Arias L.A.R., Inacio A.F., Novo L.A., Alburquerque C. and Moreira J.C., Environ. Poll., 156: 974-979, (2008), http://dx.doi.org/10.1016/j.envpol.2008.05.006







