Efficiency of Chemical Treatments on Reduction of COD and Turbidity of Deinked Pulp Waste Water
Shademan Pourmousa1 * and Somayeh Soltani Paraftabi2
1
Department of Wood and Paper Science and Technology,
Faculty of Agriculture and Natural Resources, Karaj Branch,
Islamic Azad University,
Karaj,
Iran
2
Department of Environment Science,
Science and Reseaech Branch,
Islamic Azad University, Sistan and Baluchistan,
Zahedan,
Iran
DOI: http://dx.doi.org/10.12944/CWE.8.3.09
Copy the following to cite this article:
Pourmousa S, Paraftabi S. S. Efficiency of Chemical Treatments on Reduction of COD and Turbidity of Deinked Pulp Waste Water. Curr World Environ 2013;8(3) DOI:http://dx.doi.org/10.12944/CWE.8.3.09
Copy the following to cite this URL:
Pourmousa S, Paraftabi S. S. Efficiency of Chemical Treatments on Reduction of COD and Turbidity of Deinked Pulp Waste Water. Curr World Environ 2013;8(3). Available from: http://www.cwejournal.org/?p=5208
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-08-07 |
|---|---|
| Accepted: | 2013-11-05 |
The pulp and paper industry is one of the oldest industrial sectors in the world. It is a highly capital, energy and water intensive industry with highly polluting process and requires sustainable investments in pollution control methods and equipment.
In the pulp and paper industry, a huge amount of water flowsthrough different processes. For environmental and economic reasons, the plant recycles the water as much as possible. Before recycling the water is purified to a certain degree. The chemical treatment is one of purification methods.The dosing control of chemicals is very demanding because the quality of water may fluctuate considerably and the effects of chemicals on the purification stage.1
The pulp and paper waste water contains a large amount of pollutants characterized by Biochemical Oxygen Demand (BOD),Chemical Oxygen Demand (COD), suspended solids (SS), toxicity and colorants which cause bacterial and algal slime growth, thermal impacts, scum formation, color problems and a loss of both biodiversity and aesthetic beauty in the environment.2
Several researches have been studied on biological, chemical and physicochemical treatment of pulp and paper mills waste water.3, 4 based on Thompson et al, the pulp and paper mills waste water have low BOD/COD ratio usually between0.02-0.07. Morais et al believed that the low ratio of BOD/COD makes the biological treatment methods inappropriate for pulp and paper mills wastewater.5
Waste water treatment of pulp and paper mills consumed the large amount of chemicals using alum, ferric chloride, ferric sulphate and lime through chemical processes.6 So it seems that physic- chemical processes should be interesting method for treatment of thepulp and paper mills waste water because of they are economic and based on the coagulation-flocculation process of small particles followed by an adjusted settling time.7
Deinked pulp waste wateris one of the pulp and paper conventional effluents that have especially distinctions. Therecycling rate of waste papers has steadily increased decades as parts of the effort to preserve forest resources and reduce the cost of municipal waste treatment. In this work, the effect of chemicals (poly aluminum chloride with cationic or anionic polymers) investigated on deinked pulp and paper mill waste water in order to reduction of COD and turbidity and the measurement of maximum efficiency purification.
Materials and Methods
The waste water was collected from the waste water treatment plant oftissue producing mill ofwhite mixed waste papersat Iran. The samples were taken at overflow ofphysical treatment stageof plant facility. Waste water samples were characterized and the analyses in table 1. The parameters were measured based on Standard Methods for the Examination of water and waste water (APHA 1998).8
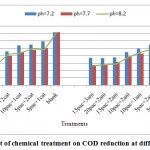 |
Figure 1: Effect of chemical treatment on COD reduction at different pH. Click here to View figure |
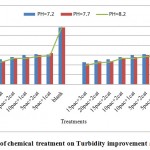 |
Figure 2: Effect of chemical treatment on Turbidity improvement at different pH. Click here to View figure |
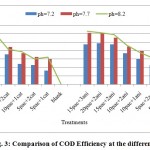 |
Figure 3: Comparison of COD Efficiency at the different pH. Click here to View figure |
All chemicals used analytically pure chemicals is commercial grade products. Anionic flocculants provided with the commercial cod of GFLOC A190 from Aquatech Company. Cationic flocculants obtained with the commercial cod of NUFLOC F10 from GIG Company. Poly aluminum chloride(PAC) provided from Iranian chemistry Company. Deion water was used to make all solutions.The chemicals were diluted to a concentration of 0.1 Percentages. Then the diluted solution was added to waste water samples.Table 2 shows the important properties of the chemicals that used in research.
Coagulation and flocculation tests were conducted using a conventional jar test apparatus. In each run, one liter samples were poured into six jars. Different dosages of chemicals(at first polyaluminum chloride and then cationic or anionic polymers ) were then added and the coagulation began with rapid mixing of 100 RPM for 2 min, followed by slow stirring of 40 RPM for 10 min. the flocks formed were then allowed to settlefor 20 min. The end of sedimentation was set at a time when no appreciable flock settlement was observed. Finally, supernatant was withdrawn with a plastic syringe from near 2 cm below the liquid- air interface for chemical analysis. All the experiments were carried out at ambient temperature of 23 -250c Decrease or increase of pH from control position to designed plan by adding of H2SO4 and NaOH was done.
Turbidity was measured by a turbid meter manufactured by Eutech (Model 2100A). Turbidity was measured by putting 10 mL of sample into turbidity cell and places it in turbidity meter to measure turbidity.Chemical Oxygen Demand was determined by the potassium dichromate method.
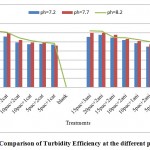 |
Figure 4: Comparison of Turbidity Efficiency at the different pH. Click here to View figure |
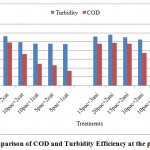 |
Figure 5: Comparison of COD and Turbidity Efficiency at the pH=7.2 Click here to View figure |
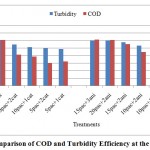 |
Figure 6: Comparison of COD and Turbidity Efficiency at the pH=7.7 Click here to View figure |
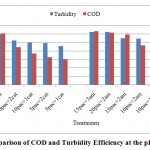 |
Figure 7: Comparison of COD and Turbidity Efficiency at the pH=8.2 Click here to View figure |
Waste water samples were treated by different dosages of poly Aluminum Chloride and Cationic or Anionic polymers at three replications. The average ofdataobtained with SPSS software 16.efficency of each treatment calculated via differences of inlet and outlet to inlet of each treatment.
Results and Discussion
Waste water distinctions at equalization tank and Overflow of physical Treatmentsummarized in table 1. Table 2 shows the important properties of the chemicals that used in the research.Comparison of results were made for treatments based on turbidity and chemical oxygen demand at variation of pH conditions are shown in figure of 1 t0 7.
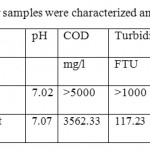 |
Table 1: Waste water samples were characterized and the analyses Click here to View table |
In order to design the best treatment for removal of COD and turbidity improvement, the research continued at three range of pH.The impact of different dosages of poly aluminum chloride with cationic or anionic polymers at three range of waste water pH, on chemical oxygen demand reduction, turbidity improvement and performance efficiency are shown at figure 1 to 7.
Based on figures, the impact of chemicals was utilized on quality of waste water clarification. the best results for COD reduction, take place at injection of 15 mg/l poly aluminum chloride with 3 mg/l cationic or anionic polymerstowaste water at PH: 8.2.at this position the performance efficiency of each treatment for COD reduction from 3562.33mg/l at blank samplesreached to1316.67-1317.83mg/l where is equal to 63.02-62.01%performance efficiency of COD removal respectively (figures 1&3).COD removal at pH: 7.2 take place with low efficiency at treatments. But the performance of COD removal increased at pH: 7.7and 8.2. Atthis range of pH,have not differences significantlyexcept at first treatments.
According to the figures, the best treatments for turbidity improvement take place attreatment of 15 mg/l poly aluminum chloride with 3 mg/l cationic polymerstowaste water atpH: 8.2.at this position the performance efficiency of the treatment for turbidity improvement reached from 117.23FTU reached to38.9 FTU that is equal66.82%turbidity improvement efficiency (figures 2&4).Turbidityimprovement at pH: 8.2 take place with high efficiency at treatments compared to other pH.
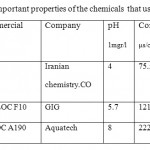 |
Table 2: the important properties of the chemicals that used in research. Click here to View table |
The trend of variations at the treatments showed, the behavior of polymers is very sophisticated at different levels of poly aluminum chloride injection. So cannot tell which kind of polymers is better than other.it seemed the application of each polymers depended to anionic and cationic traces at deinked pulp waste water effluent. The efficiency of performance at high levels of chemical consumption and upper pHgoes better than low levels. The turbidity improvement efficiency was better than COD Reduction performance at all conditions (figures 5-7).
Conclusion
Reduction of COD and turbidity has been studied using different dosages of poly aluminum chloride with cationic or anionic polymers at three range of pH. The results showed that the combination of poly aluminum chloride and polymers is more effective at coagulation and flocculation process.it can achieve 63.04 % of COD and66.82% of turbidity reduction at the optimum dosages of 15 mg/l of polyaluminum chloride with 3 mg/l cationic polymers. The waste water at this purification quality level can be used for internal process goals but without biological treatments it can’t be inject to environment and outdoors applications.
References
- Esko , k.juuso.,Dynamic simulation of water treatment in pulp and paper industry, control engineering laboratory, depts. Of process engineering, P.O.Box:3400, 90014, University of Oulu, Finland(2009).
- Pokhrel ,D. and Viraraghavan.T., Total Environment., 333:37-58(2004).
- Rintala, J., Martin,J.L.S., and Lettinga,G.,Water Sci: Technol., 24:149-160(1991).
- Rintala, J., and Puhakka,J.A.P.,A review.Bioresour.Thechnol., 47:148(1994).
- Thompson,G., J.Swain,M.Kay and Forster.C.F., Bioresour.Technol.,77:275-286(2001).
- tephenson,R.J., and Duff,S.J.B., Water Res.30:781-792(1996 .
- Kadhum M. shabeeb, Haydar A. Abdulbari, Ali A. Abbas., Al-Qadisiya J.for Eng. Sci,V:4,NO:4(2011 ).
- APHA, Standard Methods for the Examination of Water and Waste Water 20 th Edn.,American Public Health Association,Washengton, DC(1998).







