Biodegradable Hydrogels Based on Alginate for Control Drug Delivery Systems
Mohammad Sadeghi1 * , Fatemeh Shafiei1 , Esmat MohammadinAsab1 , Laleh Mansour1 and Mohammad Javad Khodabakhshi1
1
Department of Chemistry, Science Faculty,
Islamic Azad University,
Arak Branch,
Arak,
Iran
DOI: http://dx.doi.org/10.12944/CWE.9.1.16
Copy the following to cite this article:
Sadeghi M, Shafiei F, Mohammadinasab E, Mansouri L, Khodabakhshi M. J. Biodegradable Hydrogels Based on Alginate for Control Drug Delivery Systems. Curr World Environ 2014;9(1) DOI:http://dx.doi.org/10.12944/CWE.9.1.16
Copy the following to cite this URL:
Sadeghi M, Shafiei F, Mohammadinasab E, Mansouri L, Khodabakhshi M. J. Biodegradable Hydrogels Based on Alginate for Control Drug Delivery Systems. Curr World Environ 2014;9(1). Available from: http://www.cwejournal.org/?p=5827
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-01-24 |
|---|---|
| Accepted: | 2014-03-04 |
Among the diverse approaches that are possible for modifying polysaccharides, grafting of synthetic polymer is a convenient method to add new properties to a polysaccharide with minimum loss of the initial properties of the substrate. Graft copolymerization of vinyl monomers onto polysaccharides using free radical initiation has attracted the interest of many scientists. Up to now, considerable works have been devoted to the grafting of vinyl monomers onto the substrates, specially cellulose, Of the monomers grafted, acrylonitrile (MAN) has been the most frequently used one, mainly due to its highest grafting efficiency,2-3 improving the thermal resistance of the graft copolymer and also the subsequent alkaline hydrolysis of the grafting product to obtain water absorbents .
This article represents synthesis of a novel superabsorbent hydrogel based on alginate-g-polyacrylamide for control delivery system. To the best of our knowledge based on a precise survey of the Chemical Abstracts, the present paper is the first report on the preparation of a superabsorbing hydrogel through graft copolymerization of a vinyl monomer onto alginate.
Materials and Methods
Materials
Sodium alginate (chemical grade, MW 50000 and follow chemical stracture) was purchased from Merck Chemical Co. (Germany). Ammonium persulfate (APS, from Fluka) and acrylamide (AAm, Rotterdam, the Netherlands), were of analytical grade and were used as received. All other chemicals were of analytical grade.
Synthesis Procedure of the Hydrogel
A general one step preparative method for synthesis of alginate-poly(acrylamide) hydrogel was conducted as follows. alginate (0.50-1.50 g) was added to a three-neck reactor equipped with a mechanical stirrer (Heidolph RZR 2021, three blade propeller type, 300 rpm), including 40 mL doubly distilled water. The reactor was immersed in a thermostated water bath preset at desired temperature (35-70 oC). Then, at this temperature, APS was added and after stirring for 30 min (induction period) and a definite amount of acrylamide (0.5-4.5 mL) were added into the mixture. After a prescribed time (30-120 min), the obtained hydrogel was poured into methanol (200 mL) to dewater for 24 h. Then, the product was filtered and dried in an oven at 60 oC to reach a constant weight. The product was stored away from moisture, heat and light.
Drug Loading Efficiency and in Vitro Drug Release
Powdered samples (1 g ± 0.0001), with average particle sizes between 40-60 mesh (250-420 µm), were accurately weighted and immersed in an alkaline solution of ibuprofen (IBU, 0.54 g dissolved in 50 mL distilled water) at 0°C for 25 h. The swollen hydrogels loaded with drug were placed in a vacuum oven and dried under vacuum at 37°C. The loading amount of drug in the hydrogels was calculated from the decrease in the concentration of the IBU solution which was determined using a UV spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan). The loading efficiency of the alginate-based hydrogels was calculated as the ratio of the final to the initial IBU concentration.
In vitro release was carried out in duplicate by incubating 0.01±0.0001 g of the IBU-loaded hydrogels into a cellophane membrane dialysis bag (D9402, SIGMA-ALDRICH) in 50 mL of buffer solution (either pH 3 or 9) at 37°C. At specific time intervals, 1 mL aliquots of sample was withdrawn and after suitable dilution the concentration of drug released was measured by UV spectrophotometer.
The drug release percent was calculated twice using the following equation.
Released drug (%) =Rt/L×100 (1)
where L and Rt represent the initial amount of drug loaded and the final amount of drug released at time t.
Results and Discussion Synthesis and Mechanism Aspects
A crosslinking graft copolymerization of acrylamide (AAm) onto alginate was conducted using ammonium persulfate (APS) as a water soluble initiator. The persulfate initiator is decomposed under heating to generate sulfate anion-radical (Scheme1). The radical abstracts hydrogen from the hydroxyl group of the polysaccharide substrate to form alkoxy radicals on the substrate. So, this persulfate-saccharide redox system is resulted in active centers on the substrate to radically initiate polymerization of AAm led to a graft copolymer. Since a crosslinking agent, e.g. MBA, is presented in the system, the copolymer comprises a crosslinked structure. It should be pointed out that the sulfate ion-radical may also initiate AAm homopolymerization. To minimize this undesired reaction, an ²induction period² was provided in the synthesis, i.e. the initiator was introduced to the substrate before adding the monomer (See Experimental). Our preliminary studied showed low homopolymer formation (less than 4%) when the reaction was performed in the absence of crosslinker. In the presence of the crosslinker, however, the monomers are probably more intensely involved in the copolymeric network. Besides, the probable crosslinked hydrophilic homopolymer does not cause appreciable unwanted effects on absorbing properties of the final products.5-8
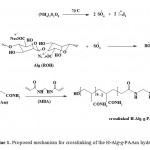 |
Scheme 1: Proposed mechanism for crosslinking |
Spectral Characterization
The grafting was confirmed by comparing the FTIR spectra of the polysaccharide substrate with that of the grafted products. Figure 1 shows the FTIR spectra of non-modified alginate and H-alginate-g-PolyAAm. The broad band at 3200-3400 cm-1 is due to stretching of –OH groups of alginate. The IR spectrum of the H-alginate-g-PolyAAm hydrogel (Fig. 1(b)) shows a new characteristic absorption band at 1680 cm-1 verifying the formation of alginate-g-PAAm. This peak attributed to C=O stretching in carboxamide functional groups of PAAm.12-13 The stretching band of –NH overlapped with the OH stretching band of the alginate portion of the copolymer
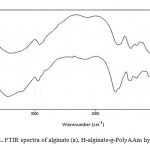 |
Figure 1: FTIR spectra of alginate (a), H-alginate-g-PolyAAm hydrogel(b) Click here to View Figure |
Scanning Electron Microscopy
One of the most important properties that must be considered is hydrogel microstructure morphologies. Figure 2 shows the scanning electron microscope (SEM) photographs of the alginate surface (Fig. 2a) and surface of the H-alginate-g-PolyAAm hydrogel (Fig. 2b). These pictures verify that the synthesized polymer in this work have a porous structure, where the pores might be induced into the hydro gel by water evaporation resulting from reaction heat. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydro philic groups of the graft copolymers.
 |
Figure 2: SEM photograph of the |
pH-Reversibility for H-Alginate-G-Poly AAm Hydro Gel
Since the hydro gels show different swelling behavior at various pHs, we investigated their pH-reversibility in solutions buffered at pH 3.0 and 9.0. A stepwise reproducible in swelling change of the hydro gel at 25 oC with alternating pH between 3.0 and 9.0 is seen in Figure 3. At pH 9.0, the hydrogel swelled up to 43 g/g due to anion–anion repulsive electrostatic forces, while, at pH 3.0, it shrunk within a few minutes due to protonation of carboxylate groups. This sharp swelling–deswelling behavior of the hydrogels makes them suitable candidates for controlled drug delivery systems. Such on-off switching behavior as reversible swelling and deswelling has been reported for other ionic hydrogels.11-12
 |
Figure 3: On-off switching behavior as reversible |
In Vitro IBU Release in the Simulated Human Gastrointestinal System
To determine the potential application of alginate-based superabsorbent containing a pharmaceutically active compound, we have investigated the drug release behavior IBU form this system under physiological conditions. The percent of released drug from the polymeric carriers as a function of time is shown in Figure 4. The concentration of IBU released at selected time intervals was determined by UV spectrophotometer. The IBU-loaded hydrogels with high degrees of drug loading (>92%) were prepared by the swelling-diffusion method. The amount of IBU released in a specified time from the H-alginate-g-PolyAAm hydrogel decreased as the pH of the dissolution medium was lowered, indicating better release in a medium with a pH much higher than that of the stomach.11
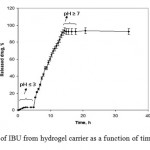 |
Figure 4: Release of IBU from hydrogel carrier |
Conclusion
A novel super absorbent hydro gel was synthesized via Graft copolymerization of acrylamide (AAm) onto alginate in an aqueous medium using a persulfate initiator. The synthesized hydrogel, H-alginate-g-PolyAAm, exhibited high absorbency in aqueous solution.
The superabsorbent hydro gels exhibited also high sensitivity to pH, so that, the reversible swelling-des welling behavior in solutions with acidic and basic pH, contributes to the suitability of these hydrogels as candidates for controlled drug delivery systems. In vitro drug-release studies in different buffer solutions showed that the most important parameter affecting the drug-release behavior of hydrogels is the pH of the solution.
References
- Buchholz, F.L. and Graham, A.T.. Modern Super absorbent Polymer Technology; Wiley, New York ,1997.
- Yazdani-Pedram, M.; Retuert, J.; Quijada, R. Macromol Chem Phys, 201, 923(2000).
- Sugahara, Y.; Takahisa, O. J Appl Polym Sci, 82, 1437(2001).
- Patel, G. M.; Trivedi, H. C. Eur Polym J, ,35, 201(1999).
- Silong, S.; Rahman, L. J Appl Polym Sci, ,76, 516(2000).
- Kost, J. In Encyclopedia of Controlled Drug Delivery; Mathiowitz, E., Ed.; Wiley: New York, Vol. 1, p 445(1999).
- Po, R.. Water-absorbent Polymers, A Patent Survey, J. Macromol. Sci., Rev. Macromol. Chem. Phys., C34: 607–661(1994).
- Kost, J. Encyclopedia of Controlled Drug Delivery. E. Mathiowitz (Ed.) Wiley, New York 1999,1, 445.
- Hoffman, A. S. Polymeric Materials Encyclopedia;, Salamone, J. C.; Ed.; CRC Press, Boca Raton, FL. 5, 3282(1996).
- Peppas, N.A. and Mikes, A.GHydrogels in Medicine and Pharmacy, CRC Press Inc., Boca Raton, Florida.,1, 27(1986).
- Gan, L.H., Deen, G.R., Gan, Y.Y. and Tam, K.C. Eur. Polym. J., 37: 1473–1478(2001).
- Mahkam, M., Doostie, L. and Siadat, S.O.R.. Inflammo Pharmacology.,14: 72–75(2006).
- Mahkam, M. and Allahverdipoor, M.. Drug Targeting, , 12: 151–156(2004).







