Evaluating Different Weeds for Phytoremediation Potential Available in Tannery Polluted Area by Conducting Pot and Hydroponic Experiments
Madhuri Girdhar1 * , Simranjeet Singh1 , Hakim Ishfaq Rasool1 , Vikram Srivastava2 and Anand Mohan1 *
1
Lovely Professional University,
Chehru,
Phagwara
2
AIDS Institute,
University of Hong Kong,
Hong Kong
DOI: http://dx.doi.org/10.12944/CWE.9.1.22
Copy the following to cite this article:
Girdhar M, Singh S, Rasool H. I, Srivastava V, Mohan A. Evaluating Different Weeds for Phytoremediation Potential Available in Tannery Polluted Area by Conducting Pot and Hydroponic Experiments. Curr World Environ 2014;9(1) DOI:http://dx.doi.org/10.12944/CWE.9.1.22
Copy the following to cite this URL:
Girdhar M, Singh S, Rasool H. I, Srivastava V, Mohan A. Evaluating Different Weeds for Phytoremediation Potential Available in Tannery Polluted Area by Conducting Pot and Hydroponic Experiments. Curr World Environ 2014;9(1). Available from: http://www.cwejournal.org?p=467/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-12-10 |
|---|---|
| Accepted: | 2014-02-26 |
Current state of environment is degrading on day to day basis because of increased anthropogenic activities and further disposal of wastesgenerated to land and rivers leading to major pollution of soil and groundwater. The industrial practices also lead to the release of various heavy metals into the soil (Mattigod and Page 1983). Pollution may be defined as the adverse effect caused due to disruption of equilibrium of an ecosystem, which further results an adverse effects on the health of organisms. The major sources of heavy metals are the practices done by the tannery industries in Indian sub continent. During the pre-tanning processes, a large amount of metal is released into the environment. Some species of plants have the ability to accumulate heavy metals into their body parts such as roots, stem and leaves. Such plants are termed as hyperaccumulators and are considered under green technology which is cost effective and ecofriendly known as phytoremediation. The extraction and inactivation of heavy metals in the soil can be done by this energy efficient technique known as phytoremediation. Phytoremediation is an emerging technology, which provides promising results in the reduction of pollution (Madhuri et al. 2014). An integrated multidisciplinary approach to cleanup the contaminated soils, phytoremediation combines the disciplines of plant physiology, soil microbiology and soil chemistry (Cunningham and Ow 1996).The development of phytoextraction technique came from the discovery of variety of wild weeds, often endemic to naturally mineralized soils that concentrate high amounts of essential and nonessential heavy metals. Rorippaglobosashows Cd hyperaccumulation as shown in the work of Yuebinget al. 2007.Phytovolatilization is the process in which the water soluble and volatile contaminants are taken up by the plant and through the process of transpiration contaminants are released into the atmosphere (Madhuri et al. 2014).The modified volatile product produced by the degradation of initial contaminants is less toxic as shown in transformation of toxic seleniumto less toxic dimethyl selenide gas (Chaudhary et al. 1998).Rhizofiltration is a cost-competitive technology in the treatment of surface water or groundwater containing low, but significant concentrations of heavy metals such as Cr, Pb, and Zn (Raskin and Ensley 2000). Hydroponic technique is also being used to accumulate and concentrate the metals in their various body parts especially roots (Flathman and Lanza 1998; Salt et al. 1995; Dushenkovet al. 1995; Zhu et al. 1999b). Phytodegradation which is also known as phyto-transformation is a process in which, the breakdown of contaminants occurs by plants through metabolic processes within the plant through plant root symbiotic associations (McGrath and Zhao 2003).
Material and Methods
Field Site, Analysis of Soil and Weeds
In this study, we investigate 3 weeds i.e. Cannabis sativa, Chenopodium album and Solanum nigrumcollected from the “Kala Sanghiya Drainage”, near Kapurthala. The area is continuously polluted by the heavy metals coming from the leather industries. The water of the drainage is continously used up by the farmers for the irrigation purposes. This research includes the metal stress of different concentration on the weeds under observation in the natural conditions by taking five different metals i.e. Chromium(Cr), Copper(Cu), Cadmium(Cd), Nickel(Ni) and Lead(Pb). The aim of this study is to assess those weeds which show least variations in their morphological characteristics underdifferent metal stress conditions in Pot experiments and Hydroponic experiments. We also analyzed the pollen fertility of the weeds under different metal concentration.
Field Demarcation and Collection of Weed Samples
Demarcation of area was done around Kala Sanghiya drainage. The area was demarcated as Polluted area(P) as Gazipur and Control areas (C) as Phiali. Cannabis sativa, Chinopodium album and Solanum nigrum were collected near the field of Kala Sanghiya drainage demarcated as (P) and the control samples from the same is collected from the other side of the road demarcates as(C).
Seed Drying and Sapling of Plants
After the sample collection, the seeds were dried under natural conditions for about 15-20 days. Saplings were prepared in the botanical garden of Lovely Professional University, (Chehru) near Phagwara.Hundred seeds were sown in the soil to germinate; out of them only forty uniform plants were allowed to grow in each pot, at a uniform distance. Seedlings were prepared after 3-4 weeks and height was approximately 2-3 cm. Sampling were prepared in around 2 months.
Preparation of Salt Concentration and Homogenization
Air-dried soil of 2.5 kg was sieved through a4 mm sieve so that no solid particles are left behind. The soil should be clean from the coarse particles. The clean soil were treated with different metal concentration i.e. standardization concentration of all the five metals at 5ppm, 10ppm, 50ppm, 100ppm, 200ppm, 300ppm and 350ppm and for comparison an unamended (control) was taken. Five different metal salts chromium chloride,copper sulphate, cadmium chloride, nickel sulphate and lead nitrate were used. They all are water soluble salts, readily dissolves in water(distilled). 50ml of water was used to dissolve the salts at different standardized concentration.
Pot Experiments
Plastic pots of 10 cm in height and 15 cm in diameter were used.Pots containing 250 grams of soil were taken and were supplemented with homogenized mixture of salt. For each weed having 5 metals and 7 concentrations were used. Pots were placed in net house shaded with transparent polythene sheet, to protect from rainwater leaching. Plants were grown under natural light and ambient temperature in order to keep all plants under conditions as similar as possible.
Pollen Fertility Analysis
Mature state plants were selected for pollen fertility experiments. Anthers were collected and preserved in carnoy’s fixator for 24 hrs and then transferred to 90% ethanol. Carnoy’s fixator was used at 6:3:1 proportion having the composition: Ethanol 600 ml, Chloroform 300ml,Acetic acid 100 ml.Glyceroacetoamine is a dye to stain the fertile pollens was used in 1:1 proportion having the composition :Glycerine 10 ml, Acetocarmine 10ml. The prepared slide was gently covered with cover slip. The slide was left for half an hour. Further it was observed under Light microscope at 100X. The slide was divided in 4 parts and pollens were counted and classified as sterile and non sterile.
Hydroponic Experiment Media Preparation
MSmedia (nutrient medium) is used (Murashige and Skoog medium) by dissolving 2.652 grams/1000ml distilled water. Chromium metal was used at additional concentrations. 50 ml glass tubes were taken and poured 50ml prepared MS media into it. Metal salt was added into the media. Chromium chloride was used for the experimental purpose and its salts concentration was added at 5ppm10ppm, 50ppm, 100ppm, 200ppm, 300ppm and 350ppm. Further, Diphenylcarbazide method was used to calculate the optical density at different prepared concentrations (Shigematsuet al. 1977).
Result and Discussion
The table 1 shows the change in the morphological characteristics of the weed Cannabis sativa under the various metal stressed conditions.The Cannabis sativa shows morphological changes as the concentration of metal increases. This shows that Cannabis sativa is the hyper accumulator of Chromium metal which shows changes in their morphological character with increase in metal concentration. In the copper metal stressed conditions, a great extent of variation wereobserved in the weed. At 100ppm metal exerts stress on the weed, the leaves area, shoot length and no. of branches decreases to large extent and the total biomass of the weed decreases with large variation in their morphological characteristic. As in the readings above, the Cadmium metal exposure to the plant do not exerts any change in the growth of plant but at 100 ppm metal exerts stress on the weed and there is a great extent of variation in the shoot length, leaf area and no. of branches of the weed. The Nickel exposure of cannabis sativa at 5 ppm shows a great variation in their shoot length, leaf area and no. of branches shows that Nickel exerts a large stress on the biomass of the plant, but at 50 ppm the shoot length increases as compared to 5 ppm, which shows that the weed can tolerate the stress up to 50 ppm and again metal stress shows variation in the morphological characteristics. The weed under Lead stress conditions shows morphological variations, weed shows least variation at 5 ppm, but as the metal concentration increases the weed shows morphological changes. In the given readings, Lead metal can exert stress maximum at 350 ppm and weed can tolerate the metal stress condition without showing much variation till 50 ppm.
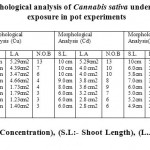 |
Table 1: Morphological analysis of Cannabis sativa under different metal exposure in pot experiments Click here to View table |
The figure 1 (a) shows that from five different heavy metals, Copper metal stress effects most to the shoot length of Cannabis sativa. It means maximum variation occurs in the morphological characteristic of Cannabis sativa at copper stressed conditions. Least variations in the morphology are observed at cadmium exposure.
In the figure 1 (b), the analysis of leaf area in the various metals stressed conditions shows that under copper exposure there is a great extent of variations observed from 5ppm to 350ppm by comparing the data, it was analyzed that the weed exert stress in copper exposure and shows variations in their leaf area as the concentration of metal increases. In cadmium and chromium exposure, appropriate results are shown i.e. maximum growth at control and decrease in leaf area from 5ppm to 350ppm. In the nickel metal stress conditions, the growth become static shows the metal have no adverse effect on the leaf area of Cannabis sativa.
The exposure of various metals on the Cannabis sativa also adversely affects the number of branches. The maximum variation again occurs in copper stressedconditions.The maximum decrease occurs in number of branches at 200ppm.In the Chromium stressed conditions, great variations occur from control to 350 ppm. At 200 ppm, the number of leaves gain increase means certain environmental factors and hormones release at this particular metal concentration. In nickel stressed state, 350 ppm favors the growth of the number of leaves as shown in the above figure 1 (c).
 |
Figure 1 (a):The figure shows the change in the shoot length of Cannabis sativa by the exposure of heavy metals Figure 1 (b): The figure shows the change in the leaf area of Cannabis sativa by the exposure of heavy metals Figure 1 (c): The figure shows the change in the number of branches of Cannabis sativa by the exposure of heavy metals Click here to View figure |
The Chenopodium album weeds with metal exposure of lead. Up to10 ppm metal does not exert any stress on the weed but at 50 ppm due to the metal stress the shoot length, leaf area and number of branches increases, it means certain hormones and other environmental factors are present which supports the morphological growth of the plant and weed get adapted in the metal stress conditions but again at 200 ppm the morphological growth decreases, which means weed is less adapted at high metal concentration and shows large morphological variations.The Chenopodium album under copper metal stress conditions, the shoot length decreases with increase in metal concentration, there is a diverse change in the leaf area as the metal concentration increases, but not much effect on the number of branches. It means, there are certain hormones and environmental factors which favour the growth. At the metal cadmium stress condition, as the concentration of metal increases, changes occurs in the morphology of the weed., overall the cadmium metal do not exert stress on the shoot of the weed, but leaf area and number of branches decreases as the metal concentration increases. In the Nickel stress condition, the plant do not show much variation in the shoot up to 50ppm, but at 100ppm the shoot length increases, it means weed is adapted up to 100ppm and show normal growth, but at 200ppm there is a adverse effect of metal concentration on the weed. High metal concentration at 200 to 350ppm exerts large stress on the weed (shoot length, leaf area and number of branches). In the lead stress conditions, due to increase in the metal concentration the morphological characteristic of the weed shows great variation. The shoot length, leaf area and no. of branches decrease with increase in the concentration of lead metal (Table 2).
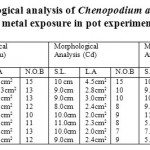 |
Table 2: Morphological analysis of Chenopodium album under different metal exposure in pot experiments Click here to View table |
In the figure 2 (a), in all the metal exposure the maximum effect is shown by the nickel metal exposure on the shoot length of the weed at 350ppm. The minimum effect is shown by the cadmium metal which shows that the toxic effect of metal on the weed is less and the weed accumulates large amount of cadmium metal without showing stress on the morphological characteristics. This shows the weed is adaptable to the metal stress environment. At 50 and 100 ppm in chromium metal exposure, enhancement of shoot length shows these conditions are favorable for the plant to grow.
The maximum stress on the leaf area was shown in nickel stress conditions as compared to other metals. The leaf area decreases to a large extent in chromium stress conditions, at 50 ppm leaf area increases shows that this is the most favorable condition for the plant to grow at maximum level. Overall favorable growth in leaf area was observed in chromium metal. This shows the weed is adaptable to these particular conditions [Figure 2 (b)].
The maximum stress on the number of branches was observed in lead stressed conditions as shown in the figure 2 (c ). Chromium metal stress showed appropriate results from control to 350 ppm. Maximum number of branches at control and minimum at 350ppm. No such effect of copper metal was observed in the weed. In cadmium metal exposure, at 5ppm exposure shows a great extent of morphological variations.
 |
Figure 2 (a): The figure shows the change in the shoot length of Chenopodium album by the exposure of heavy metals Figure 2 (b): The figure shows the change in the leaf area of Chenopodium album by the exposure of heavy metals Figure 2 (c): The figure shows the change in the number of branches of Chenopodium album by the exposure of heavy metal Click here to View figure |
The S. nigrum, when exposed to metal stress condition at different standardization concentration, the variation occurs in their morphological characteristics, in the given readings up to 100 ppm the variations occurs in the morphology(decrease in S.L, Leaf area and number of branches), but at 200ppm again the shoot of the plant increases but the leaf area is decreased to large extent, metal stress do not affect the number of branches. It means at 200ppm the weed is adapted to stress tolerant conditions. And at 300 and 350ppm least variationsoccur in the morphology, overall S. nigrum is adapted to Chromium metal stress. In the Copper stress conditions, as the concentration of metal increases, no variations occur in the shoot length and number of branches. But variation occurs in leaf area, which shows that certain factors are present in the leaf which effect the morphology of the weed, due to increase in metal concentration but overall least variation occurs and weed is adapted to copper stress conditions. In the Cadmium stress conditions, least variation occurs from 5ppm to 350ppm in the shoot length and number of branches. But large variations occur in the leaf area,whichshows that the toxic metal effectis observed in leaves only, with increase in the metalconcentration. In the Nickel stress condition, increase in metal concentration does not affect much on the morphological characteristic of plant. At 50ppm the shoot length as compared to control increases, means weed is adapted at 50ppm and again at 100ppm, metal stress conditions decreases the shoot length. Leaf area of the plant decreases, with increase in metal concentration but no metal affect is observed on the number of branches. In the Lead metal stress conditions, as the metal concentration increases, the shoot length and leaf area decreases. But no variation occurs in the number of branches. So in metal stress conditions increase in metal concentration causes a great variation in the morphological characteristics (Table 3).
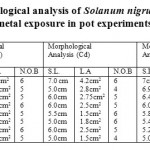 |
Table 3: Morphological analysis of Solanum nigrum under different metal exposure in pot experiments. Click here to View table |
In the figure 3 (a), the maximum stress on the morphology of plant was exerted by copper metal. With increase in metal concentration, the shoot length of the plant decreases and becomes minimum at 350ppm. Similar results were shown by the nickel metal but the metal doesnot affect so much on the morphology as compared to copper metal. At certain concentration, of the metal exposure, increase of shoot length was observed, which shows these conditions are favorable for the plant growth.
Different metal exposure on the leaf area shows that in nickel and lead stressed conditions, increase in metal concentration, decreases the biomass of the plant as shown in the figure 3 (b). Maximum stress was observed in the lead stressed conditions. In both the chromium and copper exposure, decrease in leaf area was observed at 300ppm and then increase at 350 ppm. It means in both the exposures, certain hormones and growth factors were released at 350ppm [Figure 3 (b)].
The maximum variations in the morphological characteristics were observed in nickel stressed conditions. No such variations are observed in the other metal, which shows that the plant are adaptable in that particular conditions and show normal morphological growth [Figure 3(c)].
 |
Figure 3(a): The figure shows the change in the number of branches of Chenopodium album by the exposure of heavy metals Figure 3(b): The figure shows the change in the leaf area of Solanum nigrum by the exposure of heavy metals Figure 3(c): The figure shows the change in the no. of branches of Solanum nigrum by the exposure of heavy metals Click here to View figure |
In the figure 4, pollen fertility analysis of Cannabis sativa, concluded with the outcome that the exposure of 100 ppm copper and 100 ppm chromium showapproximately similar levels of pollen fertility levels, i.e. 92. 37% and 92.18% and high pollen fertility was observed in case of cadmium exposure at 200 ppm i.e. 91.28% as compared to the chromium and copper metal exposure. The overall analysis showed that the metal exposure can affect the pollen fertility rate due to metal toxic effect on the plant.
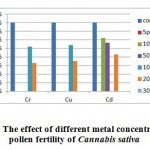 |
Figure 4: The effect of different metal concentration on the pollen fertility of Cannabis sativa Click here to View figure |
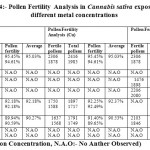 |
Table 4: Pollen Fertility Analysis in Cannabis sativa exposure to different metal concentrations Click here to View table |
In the figure 5, the pollen fertility was observed in Chenopodium album exposed to lead and copper metal at different concentrations of metal. It was observed that metal exposure at 200 ppm in case of copper,pollen fertility was 92.58% as compared to the lead exposure, where it was observed that pollen fertility was 91.54%. At 300 ppm metal exposure to Chenopodium album showed equal effect on pollen fertility i.e. 90%.
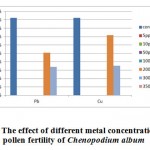 |
Figure 5: The effect of different metal concentration on the pollen fertility of Chenopodium album Click here to View figure |
In the figure 6, it was observed that metal concentration affects the pollen fertility of the weed Solanum nigrum. The data analysis concluded that upto 100 ppm, no affect on anther were observed but at 100 ppm copper exposure to plant affects the pollen fertility as compared to control, where it was 94.48% and at 100 ppm exposure of copper, it was 91.95%. The nickel exposure to plant effects adversely at higher concentration exposure, and no affect on anthers were observed at 100 ppm. At 200 ppm, the pollen fertility in copper exposure was 91.34% but no variations as compared to control were observed in nickel exposure. No anthers were observed at 300 and 350 ppm in copper exposure but the data analysis observed pollen fertility in nickel exposure at 300 and 350 ppm which was 90.75% and 89.59%
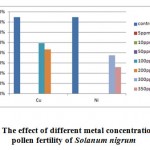 |
Figure 6: The effect of different metal concentration on the pollen fertility of Solanum nigrum Click here to View figure |
In the Hydroponic experiment (Figure 7), amount of chromium absorbed by Cannabis sativa in control and polluted samples were investigated. The ability of the plants taken from the polluted areashave more ability to accumulate chromium as compared with the Control. The graph depictsthat the polluted plants can accumulate chromium upto as compared with the Control plant, which accumulate only 32.74% of chromium from 50 ppm of active chromium available in media. The overall analysis depicts that in 350 ppm , the polluted plants accumulated 37.10% of chromium as compared with the Control plants which accumulate only 24.20% of chromium.The whole analysis concludes that the Cannabis sativa of polluted area are good hyperaccumulator of chromium metal as compared with the Control plant taken from normal agricultural land.
 |
Cannabis sativa (Control) Figure 7: Day 1(Cannabis sativa (C) Figure 8: Day 15(Cannabis sativa(C) Cannabis sativa (Polluted) Figure9: Day 1(Cannabis sativa (P) Figure 10: Day 15 (Cannabis sativa (P) Click here to View figure |
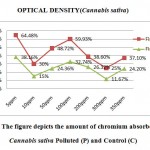 |
Figure 11: The figure depicts the amount of chromium absorbed by the Cannabis sativa Polluted (P) and Control (C) Click here to View figure |
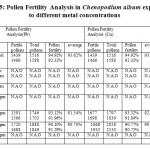 |
Table 5: Pollen Fertility Analysis in Chenopodium album exposure to different metal concentrations Click here to View table |
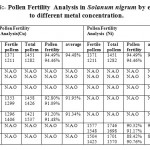 |
Table 6: Pollen Fertility Analysis in Solanum nigrum by exposure to different metal concentration. Click here to View table |
Conclusion
Agricultural practices are must for mankind and essential for the development of human race. Soil has to remain sustainable for agriculture purposes, it becomes essential to remediate the soil from the toxic heavy metals. Sustainable soil reservoir is very important for the continuum of living organisms. This particular study focuses on phytoremediation of soil from heavy metals through wild weed varieties. Four parameters are assessed during the study as shoot length, leaf area and number of branches and pollen fertility of Cannabis sativa, Chenopodium album, and Solanum nigrum. Increase in toxicological parameters along with certain level of variations was observed with increase of metal concentration. Like in S. nigrum copper metal exposure leads to decrease in the leaf area but shoot length and number of branches are least affected. It means that the weed is adapted for certain metal exposure levels. Lead metal exposure in all the weeds shows maximum toxic effects with increase in metal concentration. The best possible observations were obtained up to 50ppm in case of cannabis sativa in all the metal exposure. The pollen fertility analysis in all the weeds decreases at higher concentration of metal. The pollen fertility decreases to highest levels at 350ppm. In the hydroponic experiments, maximum toxic effect of heavy metals was seen in Cannabis sativa(P) as compared to Cannabis sativa(C). Chemical composition of nutrient solution, pH also decreases in polluted samples of Cannabis sativa as compared to control. The current set of experiments establish the basic data for carrying out metal based bioremediation protocols in various metal polluted industrial waste water with the help of wild weeds undertaken in this study. All of the weeds undertaken in the current study are capable of sufficient level of bioaccumulation and still they are capable of maintaining their growth rates and reproduction levels. This analysis needs further work to optimize the full capability of these specific weed strains.
References
- Alder T., Botanical clean up crews, Sci .News, 150: 42-43 (1996)
- Baker A.J.M. and Brooks R.R., Terrestrial higher plants which hyperaccumulate metallic elements-a review of their distribution, ecology and phytochemistry, Biorecovery, 1: 811–826 (1989).
- Chaudhary T.M., Hayes W.J., Khan A.G.E. and Khpp C.S., Phytoremediation- Focusing on accumulator plants that remediate metal- contaminated soils, Australasian journal of Ecotoxicology, 4: 37-51(1998).
- Cunningham S.D. and Ow D.W., Promises and prospects of phytoremediation, Plant Phsiol., 110: 715-719 (1996).
- Dushenkov V., Kumar P.B.A.N., Motto H. and Raskin I., Rhizofiltration: the use of plants to remove heavy metals from aqueous streams, Environmental Science and Technology, 29:1239-1245 (1995)
- Flathman P.E. and Lanza G.R., Phytoremediation: Current views on an emerging green technology, Journal of Soil Contamination, 7: 415–432 (1998)
- Ghosh M., Singh S.P., Comparative uptake and phytoextraction study of soil induced Chromium by accumulator and high biomass weed species, Applied ecology and environment research, 3(2): 67-79 (2005).
- GirdharM., Sharma N. R., RehmanH.,KumarA.,MohanA., Comparative assessment for hyperaccumulatory and phytoremediation capability of three wild weeds. 3 Biotech.DOI 10.1007/s13205-014-0194-0 (2014).
- MadejonP., Murillo J.M., Maranon T., Cabrera F., Lopez R., Bioaccumulation of As, Cd, Cu, Fe and Pb in wild grasses affected by the Aznalcóllar mine spill (SW Spain), Sci. Total Environ., 290: 105-120 (2002).
- Mattigod S.V. and Page A. L., Academic Press, Assessment of metal pollution in soil, in Applied Environmental Geochemistry, London, UK, 355–394 (1983).
- McGrath S.P., Zhao F.J., Phytoextraction of metals and metalloids from contaminated soils, Curr. Opin. Biotechnol., 14: 277–282 (2003)
- Raskin and Ensley B.D. eds., Phytoremediation of toxic metals: using plants toclean-up the environment, New York, John Wiley & Sons, Inc., 71-88 (2000)
- Reeves R.D., Baker M., Metal-accumulating plants. In: Raskin H., Ensley B.D. eds., Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment. London: John Wiley & Sons Inc., 193–230 (2000).
- Salt D.E., Blaylock M., Kumar N.P.B.A., Dushenkov V., Ensley D., Chet I., and Raskin I., Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants, Biotechnology, 13, 468-474 (1995)
- Salt D.E., Smith R.D. and Raskin I., Phytoremediation, Annual Review of Plant Physiology and Plant Molecular Biology, 49: 643-668 (1998).
- Shigematsu T., GOHDA S., Yamazaki H. and Nishikaw Y., Spectrophotometric Determination of Chromium (III) and Chromium (VI) in Sea Water, Bull. Inst. Chem.,Res., Kyoto Univ., 55 :5 (1977).
- Zhu Y.L., Bayed A.M., Quean J.H., De Souza M. and Terry, Phytoaccumulation of trace elements by wetland plants: II, Water hyacinth, Journal of Environmental Quality, 28, 339-344 (1999b)







