Physicochemical Quality of Irrigation Water in River Katsina-Ala Catchment Areas of Northern Nigeria
A. T. Ajon1 , J. T. Utsev2 * and C. C. Nnaji3
1
Department of Soil science,
University of Agriculture,
Makurdi-Nigeria
2
Department of Civil Engineering,
University of Agriculture,
Makurdi-Nigeria
3
Department of Civil Engineering,
University of Nigeria,
Nsukka
DOI: http://dx.doi.org/10.12944/CWE.9.2.10
The assessment of water qualities for irrigation in river Katsina-Ala catchment areas of Benue State was carried out. Surface water and groundwater samples from three selected catchment areas namely, Logo, Ambighir and Katsina-Ala, were collected and analyzed for physicochemical parameters. Several soil samples were also analysed for infiltration capacity. All the physicochemical parameters monitored fell within FAO specifications for irrigation purposes. Groundwater samples were found to have higher concentrations of physicochemical parameters than surface water. On the basis of hydrochemical classification, earth alkali types were dominant (100%) in both groundwater and surface water samples while the alkali type was totally absent. Assessment of the water samples for irrigation showed that the water samples posed no problems with regard to sodicity, salinity and lime deposition. However, high risk of infiltration was envisaged as a result of very low values of conductivity (0.03ds/m – 0.13ds/m). At the present, infiltration problem is minimal because of high proportion of sand (68% - 89%) in the soils but this situation may not be sustained for long. A regression model (R = 0.773) was obtained which showed that the rate of infiltration strongly depended on the sand content of soil. Both water and soil samples were found to be suitable for a wide range of irrigation.
Copy the following to cite this article:
Ajon A. T, Utsev J. T, Nnaji C. C. Physicochemical Quality of Irrigation Water in River Katsina-Ala Catchment Areas of Northern Nigeria. Curr World Environ 2014;9 (2) DOI:http://dx.doi.org/10.12944/CWE.9.2.10
Copy the following to cite this URL:
Ajon A. T, Utsev J. T, Nnaji C. C. Physicochemical Quality of Irrigation Water in River Katsina-Ala Catchment Areas of Northern Nigeria. Curr World Environ 2014;9(2). Available from: http://www.cwejournal.org/?p=6467
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-05-12 |
|---|---|
| Accepted: | 2014-07-20 |
Introduction
The demand for water has been on the increase because its uses have become more varied.Water is indispensable in man’s activities. The sources of water for usage include river, stream, lakes, ponds, rain water and groundwater such as spring water, well water, boreholes etc. In the Northern part of Nigeria, subject to the arid conditions, there has been tremendous progress in irrigation development programmes (Ahmed and Tanko, 2000). Benue State is located in the North Central geopolitical zone of Nigeria and lies within the Southern Guinea Savanna agro-ecological zone where rainfall is often erratic and inadequate in amount and distribution for production of some crops. Over 142,200ha of land are cultivated in Benue State (Ayuba et al, 2007). In Nigeria, annual rainfall varies from about 500mm in the extreme North to about 3000 mm in the south and the rainfall is high in intensity. Annual rainfall in Benue varies from about 900 to 1200 mm (Jimba and Adegoye, 2000).In Benue State, rainfed agriculture has suffered varying lengths and intensities of agricultural drought, thus necessitating irrigation in order to satisfy the moisture requirements of crops needed to meet the demands for food and fibre. The arable lands in Benue State consist of upland and fadama lands (flood plains). The upland is cultivated to many high value agronomic and horticultural crops. Fadama farming depends on rain in the wet season and residual soil moisture in the dry season. To alleviate the problem of moisture stress during the prolonged gaps between rains as well as in dry season, supplementary irrigation is provided. This is done by lifting the water from perennial surface water bodies and deep or shallow wells.Although irrigation is useful for sustaining agricultural production in any locality, it is imperative that only good quality water be used. Poor quality water affects both soil quality and crop production adversely (Bello, 2001; USDA, 2001; FAO, 1994). Considering large hectares of land which are agriculturally productive within River Katsina-Ala catchment areas, there is a felt need to encourage irrigated agriculture. This can support year round crop production on medium and consequently alleviate poverty.The river Katsina-Ala, is the tenth most important river in Nigeria (The National Atlas of the Federal Republic of Nigeria, 1978). It has a length of about 346 km and numerous tributaries (Welcomme, 1976). The main aim of the study is to investigate and evaluate the qualities of surface and ground water within the river Katsina-Ala catchment areas of Benue State, for irrigation purposes.
Study Area
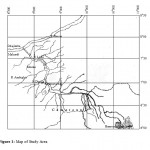
The research was carried out in three selected catchment areas of the river Katsina-Ala. The catchment areas are Logo, Ambighir and Katsina-Ala. River Katsina-Ala is located in what could be termed the Lower Benue hydro logical area, between 6050’ and 7048’N, and 8049’ and 9050’E (Fig.1). It arises from the Bamenda highlands, part of the Cameroonian mountains, (1000 – 2000m a.s.l.) meandering North-Westerly and traversing the international boundary into Benue State at Kashimbila (6055’N, 9037’E), before emptying into River Benue at Gbajimba (7048’N and 8049’N) about 160m a.s.l, (Ogueri, 2001).It has a length of about 346 km and numerous tributaries (Welcomme, 1976). River Ambighir catchment area is located at Ambighir in Gboko Local Government. The study areas are bounded by longitudes 8036’ and 8045’E and latitudes 7045’ and 8000’N, while, River Logo catchment area is located at Logo in Logo Local Government Area which is bounded by longitudes 9016’E and 9028’E and latitude 7036’ and 7050’N.The maximum elevation of river Katsina-Ala catchment area is 151.5 m above mean sea level (a.m.s.l) and minimum elevation is 121.21 m a.m.s.l. Ambighir relief ranges from 90 to 262 m a.m.s.l., and Logo ranges from about 121 to 159 m a.m.s.l. The climate of the study areas is tropical savanna.
|
|
Figure 1: Map of Study Area |
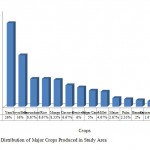
The minimum temperature is 9.70 C and maximum is 33.5 0 C. The mean monthly temperature is 27.30C. The study areas have distinct dry and wet seasons with total annual rainfall varying between about 900 and 1200mm. Rainy season starts in April and ends in October/November. The vegetation in the study areas is Guinea Savannah type, characterized by grasses with few scattered shrubs and trees. Commonly cultivated crops include yam, cassava, guinea corn, maize, millet, groundnut, soyabean, benniseed, rice, melon, and other vegetable crops. Trees crops such as mango, palm trees, citrus, cashew and other economic trees are also found in the areas. The crop mostly produced (Figure 2) is yam(26%) followed by soya bean (16%), groundnut and rice (8.67% each). Though Benue state is subject to erratic rainfall, the intense agricultural activities occurring in the state has made it the “food basket of the nation”.
|
|
Figure 2: Distribution of Major Crops |
Methodology
Water samples were taken in both wet and dry seasons. River and well water samples were collected at different locations in the three (3) catchment areas (Ambighir, Logo and Katsina-Ala). The pH of water was measured electro metrically using glass electrode pH meter (Mclean, 1965). Electrical conductivity was measured with electrical conductivity meter. Cation Exchange Capacity (CEC) of the water were analysed in the laboratory based on the Standard Methods (APHA, 1998).Sodium, potassium, chloride and boron were determined using flame photometer. Calcium, magnesium, iron and manganese were determined using atomic absorption spectro photometer (AAS) (Mclean, 1965). Bicarbonate was determined by the titri metric method using naphtalein and methyl orange as indicator (Landon, 1991).Total dissolved solids in water were determined by evaporation-drying (Chopra and Kanwar, 1991). Infiltration capacity tests were carried out in the three catchment areas of the river Katsina-Ala. Six (6) locations were determined in each catchment area. Infiltration test was done by digging 10 cm X 10 cm X 10 cm pit at each location. Water volume of 250 cm3 was poured into the pit and the time in second was taken and recorded for water transmission into the soil. This was repeated four times at each location.
Results and Discussion
Chemical Characteristics of the River and Well Water
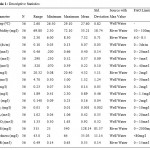
Physico chemical parameters are the most important factors used in assessing the suitability of irrigation water (Rhoades, 1977, Rogers, et al, 2003). The mean values of the various chemical constituents when compared with the FAO, (1994) water standards for irrigation were seen to fall within the ranges recommended as suitable for irrigation (Table 1 ).The turbidity of the river water ranged from 42.1 to 72.1 mg/l and from 2.3 to 30 mg/l in the wet and dry season respectively. Turbidity of the well water ranged from 8.1 to 65 mg/l and from 8 to 30 mg/l in the wet and dry seasons respectively.The pH of the river water ranged from 7.5 to 8.3 and from 7.2 to 8.1 in the wet and dry seasons respectively. The pH of the well water ranged from 6 to 7.81 and from 6 to 7.2 in the wet and dry seasons respectively.The pH of the river water was generally higher in wet season than in the dry season due to high degree of saturation with base-forming cations (Ca, Mg, K and Na) in the rainy season. In the case of well water samples, cations were expectedly leached down the soil profile thereby increasing the base concentration in the well water. All the values indicated a slightly alkaline condition, but fell within the recommended standards range of 6 – 8.5 (FAO, 1994). The pH values of river water indicated slightly alkaline condition; continuous application of this water to the soils within the study areas may be harmful. This is because of the slightly saline status of the soils in the dry season. The values of the electrical conductivity (EC) of the river water ranged from 0.03 to 0.07 ds/m and from 0.04 to 0.09 ds/m for wet and dry season respectively. The EC of the well water ranged from 0.08 to 0.13 ds/m and from 0.03 to 0.1 ds/m in the wet and dry season respectively.The values of calcium in the river water ranged from 0.24 to 0.33 meq/l and from 0.25 to 0.51 meq/l for wet and dry season respectively.Calcium content of the well water ranged from 0.23 to 0.66 meq/l and from 0.2 to 0.6 meq/l for wet and dry seasons respectively.The magnesium values in river water ranged from 0.23 to 0.33 meq/l and 0.26 to 0.42 meq/l for wet and dry season respectively. For well water, Mg ranged from 0.28 to 0.52meq/l and from 0.28 to 0.47 meq/l for wet and dry seasons respectively. Thesodium concentration in river water ranged from 0.09 to 0.21 meq/l and from 0.18 to 0.32 meq/l for wet and dry season respectively. The values of sodium in well water ranged from 0.08 to 0.39 meq/l and from 0.07 to 0.33meq/l in the wet and dry season respectively.The potassium values in river water ranged from 0.44 to 5.41 mg/l and from 1 to 20.4 mg/l in the wet and dry season respectively. The K values in the well water ranged from 1 to 16 mg/l and from 0.08 to 7mg/l in wet and dry seasons respectively.The values of boron in the river water ranged from 0.46 to 0.76 mg/l and from 0.09 to 0.14 mg/l for wet and dry seasons respectively. In well water, boron values ranged from 0.1 to 0.3 mg/l and from 0.07 to 0.23 mg/l for wet and dry season respectively.The values of Fe ranged from 0.41 to 1.1 mg/l and from 0.41 to 2.3 mg/l for river and well water respectively. Mn ranged from 0.11 to 0.18 mg/l and from 0.09 to 0.23 mg/l for river and well water respectively. Sulphate values in the river water ranged from 0.07 to 0.92 meq/l and from 0.4 to 0.7 meq/l for wet and dry season respectively. In the well water, the values ranged from 0.1 to 1.06 meq/l and from 0.09 to 1 meq/l for wet and dry season respectively.The values of bicarbonate in the river water ranged from 0.60 to 1.01 meq/l and from 0.61 to 1.52 meq/l for wet and dry seasons respectively. The bicarbonate in the well water ranged from 0.59 to 1.63 meq/l and 0.3 to 0.96 meq/l for wet and dry seasons respectively.The values of total dissolved solids in water ranged from 87 to 340 mg/l and from 25 to 200 mg/l for river and well water respectively.
|
|
Table 1: Descriptive Statistics |
|
|
Table 2: Correlation of Physico chemical Parameters |
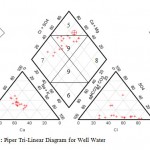
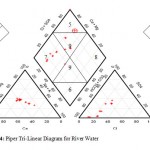
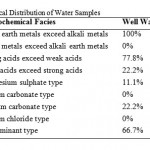
The water samples were further analyzed by generating plots of Piper trili near diagrams. Cations were plotted of the left triangle while anions were plotted on the right triangle. The diamond shaped field was then obtained by projecting points from the two triangles to meet in the diamond shape (Figures 3, 4). The dominant hydro chemical type was the Ca-Mg (earth alkali metals) type recording 100% for both well and river water samples (Table 3) followed by the SO42--Cl- type recording 77.8% and 50% for well and river water samples respectively. The Na – K (alkali metals), sodium carbonate and sodium chloride types were totally non-existent (0%) in both well and river water samples. The proportions of both the HCO3-+CO32- type and the calcium carbonate type in river water samples (50% each) were more than twice their proportions in well water samples (22.2% each).River water is in contact with the atmosphere and this makes it possible for carbon dioxide to dissolve in water to form bicarbonate ion. This does not readily occur in groundwater which is shielded from the atmosphere. The proportion of well water samples belonging to no particular hydro chemical facies was roughly twice the proportion of river water belonging to no particular hydro chemical facies. This can be attributed to the fact that river water experiences more turbulent mixing as it flows while groundwater experiences less mixing.
|
|
Figure 3: Piper Tri-Linear Diagram for Well Water |
|
|
Figure 4: Piper Tri-Linear Diagram for River Water |
|
|
Table 3: Hydrochemical Distribution of Water Samples |
Evaluation of Water Quality for Irrigation
For irrigation purposes, both quantity and quality of water are of equal importance (Sangodoyin and Ogedemgbe, 1991). The quality of water is assessed based on its intended use. The quality of water is highly dependent on its source and anthropogenic activities occurring around it. For irrigation water, the concern is not just its suitability for crops productivity; its effect on agricultural soil and irrigation systems must also be taken into consideration. The suitability of irrigation water (SIW) is expressed as:
SIW = f (Q, S, P, C, D)
Where; Q = quality of irrigation water, S = soil type, P = salt tolerance characteristics of plant, C = climate, D = drainage characteristics of the soil.
Risk of Sodi city
Evaluating the concentration of sodium in irrigation water is crucial because of its high solubility in water and the negative effects associated with sodium in irrigation water. Excess sodium content in irrigation water can affect plant growth and affect soil permeability by damaging soil structure. In extreme cases, toxicity to plants becomes a possibility, hence the need to evaluate the sodium content of irrigation water. Percent sodium is obtained as
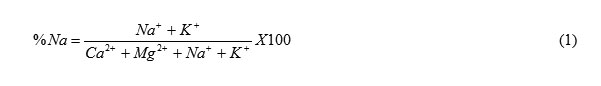
The concentration of sodium in the water samples was generally very low. All the samples had sodium concentration within the range recommended by FAO (1994) while percent sodium values were below 3meq/l which means that sodicity problem is not expected. Sodicity is the presence of excess sodium in soil (Singh, 2000). Sodicity causes swelling and dispersion of clay particles, surface crusting and pore plugging (Bauder et al, 2011) both of which aggravate infiltration problems. This condition makes it difficult for plants to get enough water. Excess sodium also causes problem by competing with plants for nutrients since it is extremely reactive. In irrigation systems using sprinklers, excess sodium can cause damage to foliage.Because the ratio of Ca/Mg is greater than one, the potential effect of sodium is reduced.
Salinity Problem Evaluation
Irrigation water contains a mixture of naturally occurring salts. Soils irrigated with this water will contain a similar mix but usually at a higher concentration than in the applied water (Oster and Rhoades, 1983). Generally, the electrical conductivity values for the water samples were low. Electrical conductivity is a measure of total dissolved solids. For the river water, electrical conductivity in the dry season was higher than that of the wet season. River water quality is often related to flow. The dilution due to runoff in the rainy periods usually keeps total salt concentration low (Ochtman and Debele, 1975). For the well water, electrical conductivity values were higher in wet season compared to the dry season. Based on the FAO (1994) standards, the value of 3.0 ds/m is the upper limit of conductivity for irrigation water. For proper evaluation of the salinity problem posed by irrigation water, a combination of sodium adsorption ratio (SAR) and electrical conductivity must be considered (Rhoades, 1977). SAR is used to evaluate sodium hazard and is determined as follows:

Salinity hazard was evaluated using USSL (1954) classification of irrigation water (Figure 5). The three slanting lines can be plotted using the following expressions:
Upper curve S=43.75-8.87LogC .......(3)
Middle curve S= 31.31-6.66LogC .......(4)
Lower curve S= 18.87-4.44LogC .......(5)
Where S = sodium adsorption ratio and C = electrical conductivity.
Figure 5 shows that all the samples analyzed were of excellent quality (class C1-S1) with regard to salinity hazard. Table 6 shows that both river water and well water sampled are very good for irrigation as far as salinity is concerned. The values of pH, EC, Ca, Mg and Na indicate that the water has no salinity problems. The water in the three catchment areas is, therefore, good quality for irrigation. This is significant because saline water increases the osmotic exertion required for plants to absorb water from the soil. Hence as salinity increases, less water becomes available for plant uptake even when there is adequate water in the soil. Excess salt in irrigation water can further cause reduced plant yield, desiccation of plant leaves and discoloration of fruits with consequent reduction in market value. The problem of salinity in irrigation water can be corrected by leaching and dilution with water of good quality.
|
|
Figure 5: Irrigation Water Quality |
Risk of Infiltration
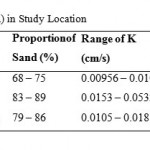
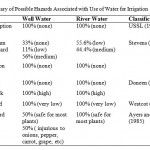
The soil problems most commonly encountered and used to evaluate water quality are those related to salinity, water infiltration rate and toxicity problems (Rogers et al., 2003; FAO, 1994; Ayers and Westcot, 1994; USDA, 2001; Yakubu et al., 2006). Irrigation water of high salinity content can cause salt accumulation in soils which leads to soil structure problems. SAR and electrical conductivity can be used to assess the risk of infiltration as suggested by Ayers and Westcot (1985). As previously noted, the water samples pose no salinity threats but Table 5 shows that they pose serious infiltration problems. All the water samples analyzed had very high risk of water infiltration problem. However, the extent of infiltration risk resulting from irrigation waterdepends on soil characteristics with the risk being higher as clay content of the soil increases. Despite the high risk of infiltration problem posed by the water, the soils have not yet developed infiltration problems because of the high sand content (Table 4). Table 4 shows the results of the infiltration capacity tests carried out in Logo, Ambighir and Katsina-Ala respectively. The values of the infiltration capacity fell within the range suitable for a wide range of crops and irrigation. Table 4 shows that the soils had high values of infiltration capacities varying from 9.56 x 10-3 cm/s to 5.32 x 10-2 cm/s. The infiltration capacity was found to be proportional to the percentage of sand in soil and the clay content increased with depth.A multiple regression model (R= 0.773) relating infiltration capacity to proportion of sand was obtained (Equation 6).
K = 0.1 Sand + 0.076 Silt - 0.089 .......(6)
Kis the infiltration capacity while sand and silt are the proportions (fraction) of sand and silt respectively. Including clay content in the model did not improve the model, hence it can be inferred that clay content should not be of concern when present in the quantities found in these soils. Infiltration problems can also result from extremely low electrical conductivity (too little dissolved salt). The range of conductivity observed in the water samples (0.03ds/m to 0.13ds/m) can cause disintegration of soil aggregates.
|
|
Table 4: Infiltration Capacity (K) in Study Location |
Permeability problem related to water quality may occur when the rate of water infiltration into and through the soils is reduced by the effect of specific salts or lack of salts in the water to such an extent that the crop is not adequately supplied with water and yield is reduced.
|
|
Table 5: Summary of Possible Hazards |
Residual Sodium Carbonate (RSC)
Residual sodium carbonate is estimated as the difference of carbonate plus bicarbonate and calcium plus magnesium. The RSC for well water ranged from -0.573 – 1.594meq/l while that of river water ranged from 0.364 – 1.365meq/l. RSC values below zero are considered safe for irrigation while values above zero render soil susceptible to structural problems. Soil structural problems develop when, as a result of high RSC, calcium is lost from the soil by precipitation (lime deposition). Table 5 shows that all river water samples posed varying degrees (55.6% low and 44.4% medium) of soil problem as a result of high RSC while 33% of well water sample posed no risk at all.This attests to high amount of calcium in well water compared to river water and could be due to the presence of limestone within the lithology. As groundwater flows through the limestone formation, the calcium content increases as a result of cation exchange.Though the RSC values of the water samples indicated the possibility of structural problems, Table 5 shows that lime deposition will not occur. Lime deposition is caused by evaporation, loss of carbon dioxide as gas, increased temperature and increased pH. Lime deposition reduces the marketability of crops by leaving white patches on leaves and fruits, plugs irrigation systems and reduces nutrients available to plants by precipitation or reduced solubility.
Other Hazards
If the values of the water sample analyses summarized in Table 1 are taken at face value, all parameters without exception in all the locations fell within FAO(1994) standards. The most common toxicity problem is from chloride in irrigation water. This is because chloride is not adsorbed or held back by soils. Therefore, it moves readily with the soil-water (Maas, 1984). Generally, chloride concentration in both river and well water was below the 30meq/l safe limit (FAO, 1994) for both wet and dry seasons. The low chloride concentration might be due to the presence of basalt which prevents marine cretaceous sediments from getting in touch with the fresh water. However, Table 4 shows that the possibility of chloride hazard exists. All the river water samples analyzed did not pose any chloride hazard while 50% of well water samples are likely to be injurious to such crops as onions, pepper, carrot and grape. High chloride concentration corrodes plant leaves and fruits. This can be prevented by dilution and by avoiding contact between leaves and water during irrigation.Trace elements posed no risk at all since they were allthan 100 µg/l as noted by Pratt, 1972.
Conclusion
An assessment of the quality of water at some locations along the river Katsina-Ala catchment areas of Benue State was carried out to determine the suitability of the water for irrigation purposes. The river and well water qualities were found to be suitable for wide range of irrigation, as salinity, permeability, toxicity and miscellaneous quality related parameters fall within the tolerable limit as recommended by the FAO (1994).Hence, hazards associated with the use of both surface water and groundwater for irrigation are presently very low. However, soil structure problems are likely to develop if proper management practices are not initiated.
References
-
Agbede, I.O., and Adegoye, M.S. (2003) Assessment of the quality of borehole water in Benue State, Nigeria. J. Agric. Sci. and Technology, Vol. 13, No 1, pp 38 – 50.
-
Ahmed, K., and Tanko, A.I. (2000). Assessment of water quality changes for irrigation in the river Hadejia catchment. J. Arid Agric.,Vol 10, pp 89-94.
-
Ayers, R.S., and Westcot, D.W. (1994).Water quality for agriculture. FAO Irrigation and Drainage Paper 29 Rev. 1 FAO of the UN,Rome.
-
Ayuba, S. A., Akamigbo, F. O. R., Itseghe, S. A (2007). Properties of soils in River Katsina-Ala Catchment Areas, Benue State, Nigeria. Nigerian Journal of Soil Science, Vol 17, pp 24 – 29.
-
Bauder, T. A., Waskom, R. M., Sutherland, P. L. and Davis, J. G. (2011) Irrigation water quality criteria, Colorado State University Extension, Fact Sheet No 0.506
-
Bello, S.(2001). Water quality and soil characteristics of wetland in Sokoto metropolis. Unpublished B.Sc Project Report. Department of Soil Science and Agric Engineering,UsmanDanFodioUniversity,Sokoto,Nigeria. 69pp
-
Chopra, S.L., and Kanwar, J.S. (1991). Analytical Agricultural Chemistry. 4th Edition,New Delhi,India: Kalyani publishers.
-
Doneen, L. D. (1954) Salinization of soils by salt in irrigation water. Trans. Amer. Geophy. Union Vol, 35 pp 943 – 950.
-
FAO, 1994. Water Quality for Agriculture. FAO Irrigation and Drainage Paper. 29 Rev. 1.Rome: FAO.
-
Jimba, S.C., and Adegoye, M.S. (2000). An assessment of the reliability of the handdug wells in River Bar catchment areas for small-scaleirrigation, In: The Proceedings of the 26th Annual Conference of Soil Sci. Society ofNigeria.
-
Landon, J.R. (1991) Booker Tropical Soil Manual. A Handbook for Soil Survey and AgriculturalLand Evaluation in the Tropics and Sub-Tropics. New York, USA. John Wiley and Son Inc. 474pp.
-
Maas, E. V. (1984). Salt tolerance of plants. In: The Handbook of Plant Science in Agriculture. B.R. Christie (ed). Boca Raton, Florida.CRC Press,
-
Mclean,E.O., (1965). Aluminum. In:C.A., Black, D.D. Evans , J.L. White, L.E. Ensminger, and F.E. Clark,(eds.) Methods of Soil Analysis. Part2. Chemical and mineralogical proper ties. No. 9. Madison, Wis.USA. Am: Soc. Agon.
-
Ogueri, C. (2001). The fish abundance and potentials of riverKatsinaAla,Nigeria. J. Agric., and Related Sciences, Vol. 1, No 1, pp 24 – 30.
-
Oster, J.D; and Rhoades, J.D. (1983). Irrigation with saline water. In: Soil and Water Newsletter. University of California Cooperative Extension, Vol. 56, pp1-3.
-
Pratt, P.F. (1972). Quality criteria for trace elements in irrigation waters. California Agricultural Experiment Station, p 46
-
Rhoades, J.D., and Merrill, S.D.(1976). Assessing the suitability of water for irrigation: theoretical and empirical approaches. In: Prognosis of Salinity and Alkalinity. FAO Soil Bulletin 31.Rome, FAO,Rome. 69-110.
-
Rhoades, J.D. (1972). Quality of water for irrigation. Soil Science, Vol. 113, pp 277-284.
-
Rhoades, J.D. (1977). Potential for using saline agricultural drainage waters for irrigation. Proc. Water Management for Irrigation and Drainage. ASCE,Reno,Nevada. 20-22 July 1977. pp. 85-116.
-
Rogers, D.A; Lamm, F.R; and Alam, M. (2003). Subsurface drip irrigation system (SDI) water quality assessment guidelines. Irrigation Management Series.KansasStateUniversity,Manhattan,Kansas.
-
Sangodyin, A.Y., and Ogedengbe, K. (1991). Subsurface water quality and quantity from the stand point of irrigation and livestock. J. Environ. Studies, Vol. 3, pp 251-262.
-
Singh, B.R. (2000). Quality of irrigation Water in Fadama Lands of Northwestern Nigeria. I. Ground and surface water in Kebbi State Nig. J. Basic and Apl. Sci., Vol. 9, pp 133 – 148.
-
USDA, 2001. Guidelines for Soil Quality Assessment in Conservation Planning.WashingtonDC,United StatesDept. of Agriculture, Natural Conservation Service. Soil Quality Institute.
-
USSL (1954) Diagnosis and improvement of saline and alkali soils. USDA Agr. Handbook No. 60, Washington D. C.
-
Welcomme, R.L.(1976). Some general and theoretical considerations on the fish of African rivers. T. Fish Bio., Vol. 8, pp 351 364.
-
Yakubu,M, Bello,S, Noma,S.S. and Danmowa,N.M. (2006). Quality of irrigation water and soil characteristics of Fadama lands in Sokoto Metropolis. In: Proceedings of the 30th Annual Conference of the Soil Science Society of Nigeria, pp 333- 341.