The Impact of Nanosilver Particles on Foot -Odor Producing Microbes
Bassam Mashat and Abdel Hameed *
1
Department of Environmental and Health Research,
the Custodian of the Two Holy Mosques Institute for Hajj and Umrah Research,
Umm Al Qura University,
21955
Makkah
Saudi Arabia
DOI: http://dx.doi.org/10.12944/CWE.9.3.09
Nanosilver applications have been implicated in paint, plastic, textile and medical industries. Nanosilver particles absorb sweat and in turn avoid pungent smell of feet. The present study aims to determine the antibacterial activity of Ag-coated different sock fabrics and comparing their efficacy in reducing foot-borne resident bacteria. Synthesize of nanosilver particles was performed in various concentrations by wet reduction method using silver nitrate and tri-sodium citrate. Coating of different sock fabrics, cotton, nylon, and mixed cotton and nylon, was carried out by exposing these fabrics to various silver concentrations for 24 hrs. The antibacterial efficacy of the nanosilver-finished fabrics was checked by zone inhibition test and antibacterial test. The coated nylon fabric showed better antimicrobial activity than other fabrics. There was no significant difference found between antibacterial activity and type of fabric. Klebsiella pneumoniae exhibited the smallest inhibition zone, and the inhibition zone increased with increasing silver concentration. The inhibition zones varied according to bacterial species, each species had its own minimum inhibition concentration. The percentages of reduction ranged between 18-80%. Sarcina lutea was highly sensitive to nanosilver particles. The effect of temperature, relative humidity, dirt and oil on the antibacterial activity of silver coated fabrics should be studied in the future.
Copy the following to cite this article:
Mashat B, Awad A. H. A. The Impact of Nanosilver Particles on Foot -Odor Producing Microbes. Curr World Environ 2014;9 (3) DOI:http://dx.doi.org/10.12944/CWE.9.3.09
Copy the following to cite this URL:
Mashat B, Awad A. H. A. The Impact of Nanosilver Particles on Foot -Odor Producing Microbes. Curr World Environ 2014;9(3). Available from:http://www.cwejournal.org/?p=7690
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-07-09 |
|---|---|
| Accepted: | 2014-08-29 |
Introduction
The antimicrobial activity of the silver ions was first identified in the 19th century, and colloidal silver was accepted by the US Food and Drug Administration (FDA) as being an effective for wound management in the 1920s.1,2 The antibacterial effects of Ag- salts have been noticed since antiquity.3 Silver is an antifungal and antibacterial agent for antibiotic resistant microbes,4 it has beneficial healing and anti-disease properties,5 and it prevents infection, and anti-inflammatory.6
Nanotechnology is a rapidly growing science of producing and utilizing nano-sized particles. Nanosilver particles have large specific surface area, thus increasing their contact time with bacteria and fungi, and vastly improving its bactericidal and fungicidal effectiveness.7 The use of metal nanoparticles for water disinfection is relatively new.8,9 Nanosilver coating surface of textile or footwear is one approach to produce highly active surfaces to have UV blocking, antimicrobial, and self cleaning properties.
Suppression of microbial growth is accomplished by exposing the feet to air to enhance evaporation and reduce moistures' growth-stimulating effect. The resolution of a severe infection requires prescription drugs, salves, or foot soakings. Baking soda, basil oil, tea tree oil, sage oil, and clove oil have been reported to inhibit the growth of both aerobic bacteria and yeast-mold-fungi.10,11 Several studies have been carried out on bactericidal activity of nano particles and their applications in plastic, health, textile and paint industries.12-14 Silver is a medically proven natural antibacterial agent, which acts to kill most bacteria that cause foot odor and other sweaty foot problems.15 Silver ions are passed from the silver fibers to infiltrate odor emitting bacterial cells and bond with their DNA chains.7 Silver nanoparticles destabilize plasma membrane potential and depletion of levels of intercellular adenosine triphosphate by targeting bacterial membrane resulting in bacterial cell death.16
The common skin microbes rapidly proliferate in a moisture-rich and enclosed environment resulting in limited or no athletic participation. Hot weather, sweating, exercise, and shoes generate a moisture-rich environment that stimulates overgrowth of both aerobic bacteria and fungi. The present study aims to determine the antibacterial activity of Ag-coated different sock fabrics and comparing their efficacy in reducing foot-borne resident bacteria.
Materials and Methods
Synthesis of Nanosilver Particles
Chemical wet reduction method was used to synthesize nanosilver particles. The synthesis of Ag-citrate was done according to the literature procedure.17 Breifly: 25 mL of 0.005 molar (M) stock solution of silver nitrate in water was diluted to 125 mL, and heated until it begins to boil. Then 5 mL of 1% tri-sodium citrate solution was added; heating continued until the color was pale yellow. The yellowish brown color indicates formation of nanosilver particles. The solution was cooled at room temperature, and serial dilutions were prepared, (0.005 M, 0.01 M, and 0.05 M).
Coating of Sock Fabrics with Nanosilver Particles
Pieces of 20 x 20 mm size (400 mm2 ±0.1mm2) of each sterilized sock fabrics, nylon, cotton and mixed cotton and nylon, were soaked into various concentrations of Ag-citrate overnight. The fabric pieces were washed with sterilized distilled water to remove any adsorbed ion particles, and dried at 120°C in a hot air oven for 30-60 min18.
Evaluating the Antimicrobial Activity of Nanosilver Particles Against Bacterial Species
The antimicrobial activity of nanosilver particles coated sock fabrics was studied against Sarcina lutea, Pseudomonas aeruginosa, Staphylococcus epidermidis, and Klebsiella pneumoniae. These bacteria are frequently isolated from human skin. The tested bacterial species were inoculated in test tubes containing trypticase soya broth (Hi-Media Laboratories, India) medium, and incubated at 37oC for 18 h. The culture suspensions were serially diluted in sterilized distilled water reaching a final concentration of 104 colony forming unit per milliliter (CFU/mL).
The Antimicrobial Efficacy of Nanosilver Particles was Evaluated by the following tests
Zone Inhibition Test
Antimicrobial activity of the prepared nanosilver particles was confirmed by zone inhibition test.19 A sterile cotton swab was dipped into the culture suspensions, and streaked onto the surface of Muller Hinton agar medium. Square pieces of both the coated (test) and uncoated (control) sock fabrics were gently placed on the Muller Hinton agar. This procedure was repeated for all the tested bacterial species with different sock fabrics and Ag- concentrations. The plates were incubated at 37oC for 24 h, and the zone of inhibition was measured.20
Antimicrobial Test
The coated (test) and uncoated (control) fabrics with 400 mm2 ± 0.1 mm2 diameters were placed in a 250-ml sterile flask, and allowed to absorb 0.5 ml of different bacterial culture suspensions. The flasks were incubated at 37oC for 24 h. After incubation, 20 ml of sterile distilled water was added into the flasks and shaken vigorously for 3 min. Serial dilutions up to 10−3 were prepared. Aliquots, 0.2 ml, of the original sample, and its serial dilutions were spread-plated, in triplicate, onto the surface of nutrient agar for counting of the tested bacteria. The percentage of reduction of viable organism was calculated according to Shastri and his coauthors.7
Isolation and Identification of Foot-Borne Bacteria
The samples of sock and foot skin were collected from 10 volunteers by swab sampling technique. The volunteers were healthy people, without wounds, diabetic, and athlete's foot infections; but suffer from pungent foot odors. The swabs were suspended in phosphate buffer solution, and shaking vigorously for 15 min. Serial dilutions were prepared, and aliquots (0.5 ml) were spread-plated onto the surface of nutrient agar medium. The bacterial plates were incubated at 370C for 48 h.
Three to five isolates of different colony morphology which appear in more than 5% of the nutrient agar medium plates were picked up, purified and subcultured for further identification. Bacterial isolates were identified using Gram stain, oxidation fermentation, oxidase and catalase tests described in the Bergey’s Manual of Systematic Bacteriology.21
Statistical Analysis
ANOVA (one-way and post hoc double comparison tests) were used for determining antibacterial activity on bacterial growth with various Ag coated fabric concentrations. Statistical analysis was performed using SPSS 18 (PASWStatistics 18). P≤0.05 was considered as significant.
Results and Discussion
Identification of Bacterial Isolates
A total of 315 bacterial isolates belonging to 14 genera were identified in Table 1. Kocuria and Sarcina were the dominant Gram positive cocci, and Gram-negative bacteria represented by Klebsiella, Escherichia coli, Pseudomonas and Acinetobacter. Pseuddomonas species were the dominant Gram negative bacterial species isolated. Gram positive bacteria were the dominant isolates, because they are associated with skin scales and every day human activities. Most of the isolated bacterial genera are non-medical important for the healthy people, however some genera produce pungent foot odor and play an important role in wound infection. Gram negative bacteria may be dangerous if they are detected in high concentration.22
|
|
Table 1: The percentages of bacterial genera |
Nanosilver Oarticles
Nanosilver particles were produced by chemical reduction of silver nitrate (AgNO3) and trisodium citrate according to the following reaction
4Ag+ + C6H5O7Na3 + 2H2O → 4Ag0 + C6H5O7H3 + 3Na+ +H+ +O2
The surface of sock fabric is highly porous which may results in homogeneity of nanoparticles inside fabric.
Silver coated fabric
is anti-bactericidal to a wide range of pathogens, absorbs sweat and helps eliminate unpleasant foot odor by killing bacteria.23
Antibacterial Efficacy of Ag-Coated Fabric Against Foot Borne Bacteria
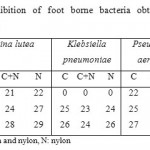
The antibacterial activity varied depending on the type of sock fabric, Ag-concentration, and bacterial species (Table 2). The inhibition zones of Ag-coated sock fabrics on bacterial growth are shown in figures 1-3. The one-way descriptive analysis of variance (ANOVA) showed no significant differences between inhibition zones with Ag-coated cotton, nylon, and mixed cotton fabrics (p≥0.05). However, nylon fabric exhibited better antimicrobial activity (Fig. 3). Ag-coated sock fabrics exhibited the highest antibacterial activity against Pseudomonas aeruginosa and the lowest against Klebsiella pneumoniae.
|
|
Table 2: Zones of inhibition(mm) of foot borne bacteria obtained with sock coated nanosilver-particles |
|
|
Figure 1: Inhibition zones obtained by Ag-cotton |
|
|
Figure 2: Inhibition zones obtained by Ag-coated |
|
|
Figure 3: Inhibition zones obtained by Ag-coated |
The Effect of Ag-Concentration on Bacterial Growth
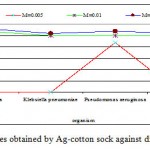
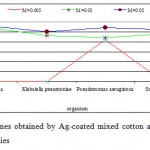
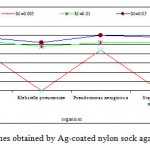
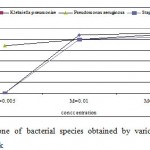
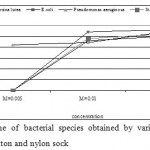
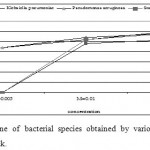
The antibacterial activity varied with Ag-concentration. Increasing concentration of Ag-coated sock fabrics over a range of 0.005 M to 0.05M increased inhibition zone (Figs. 4-6). Pseudomonas aeruginosa inhibited with Ag-coated cotton and mixed fabrics at concentration of 0.005M. Pseudomonas aeruginosa was found to be the most sensitive to nanosilver particles. Ag-coated nylon fabric effectively inhibited Sarcina and Pseudomonas (Fig. 6). Klebsiella pneumoniae exhibited more resistant to nanosilver; however S. aureus and Pseudomonas aeruginosa were more sensitive. This can be attributed to the cell wall structure of Klebsiella. Gram-positive and Gram-negative bacteria differ in their membrane structure, the thickness of the peptidoglycan layer. Klebsiella tends to be rounder and thicker than other members of the Enterobacteriaceae family. Klebsiella forms a capsule or slime layer.24 These results suggested that the antimicrobial activity of nanosilver particles depended on characteristics of bacterial species, and every species had its own minimum inhibition concentration. In this study Pseudomonas and Sarcina were inhibited at the lowest Ag-concentration.
|
|
Figure 4: Inhibition zone of bacterial species obtained |
|
|
Figure 5: Inhibition zone of bacterial species |
|
|
Figure 6: Inhibition zone of bacterial species obtained |
Antibacterial Test
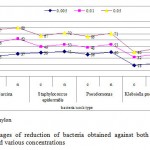
The degree of antibacterial activity was carried out against bacterial species with Ag-coated and uncoated cotton and nylon sock fabrics (Fig. 7). Ag-coated nylon fabric showed higher rate of killing than Ag-coated cotton fabric. However non significant difference was found between Ag-coated nylon and cotton sock fabrics (P> 0.05). The rate of killing increased gradually with increasing Ag-ion concentration. A significant difference (P<0.05) was found between Ag-coated and uncoated sock fabrics. Ag-coated cotton and nylon sock fabrics effectively inhibited Sarcina lutea, with increasing Ag-concentration, the percentage of reduction reached 77% and 80%, respectively. Klebsiella pneumoniae exhibited low killing rate, with percentages of reduction ranged between 18-46%.
|
|
Figure 7: Percentages of reduction of |
Gupta and Cooper25 observed that the growth of bacterial colonies around silver-loaded cotton fabric was inhibited. One potential advantage of silver nylon is its ability to release silver ions continuously over the entire time period.26 This confirms the greater inhibition zones of nylon sock fabric coated with nanosilver particles. Hong et al.23 evaluated the antibacterial activities of the heat-treated PVA/AgNO3 nano-fibres against S. aureus and K. pneumonia. They found a reduction in the number of colonies of S. aureus and K. pneumoniae (> 99.9%) after 18 h of incubation. These results are not agreement with the results in the present study, this difference is attributed to the materials and techniques were used.
The mechanism of the inhibitory effect of Ag ions on microorganisms is partially known. Some studies have reported that the positive charge on the Ag- ion is crucial for its antimicrobial activity through the electrostatic attraction between negative charged cell membrane of microorganism and positive charged nanoparticles.27 The antimicrobial activity of nanosilver particles on Gram-negative bacteria depended on Ag nanoparticle concentration, and formation of pits in the cell wall of bacteria.28 The effectiveness of silver compound as an antiseptic is based on the ability of the biologically active silver ion to irreversibly damage key enzyme systems in the cell membranes of pathogens,29 and depletion of levels of intracellular adenosine triphosphate by targeting bacterial membrane.16
Nanosilver particles effectively inhibit growth of various microorganisms, making them applicable to diverse medical devices and antimicrobial control systems. Coating of socks fabric with nanosilver particles can be used as an effective way to combat foot-borne pathogens and thereby reducing discomfort like foot odor, and athlete’s foot, without toxic or allergenic effects.
Conclusion
Nanosilver proved to be effective in inhibiting bacterial growth. Ag-coated sock fabrics sufficiently inhibited bacterial growth. Ag- coated fabric acts as antimicrobial agent killing foot-odor producing bacteria. The percentage of reduction increased gradually with increasing silver concentration, reached ~80% for Sarcina lutea. Ag-coated nylon sock fabric exhibited greater antimicrobial activity than cotton fabric. Klebsiella pneumoniae was highly resistant to nanosilver particles. Coating of socks fabric with nanosilver particles can be used as anti-odor, and to combat foot-borne bacteria.
Acknowledgment
This work was funded by Scientific Research and Revival of Islamic Heritage Institute, Umm Al Qura University, Makkah, Saudi Arabia
References
-
Demling R.H. And Desanti L., WOUNDS; 13(1), 5 (2001).
-
Chopra I., The Journal Of Antimicrobial Chemotherapy 59 (4), 587(2007).
-
Silver S., Phung L.T., Annu Rev Microbiol, 50, 753 (1996).
-
Niakan S., Niakan M., Hesaraki S., Nejadmoghaddam M.R., Moradi M., Hanafiabdar M., Allamezadeh R., Sabouri M Jundishapur Journal Of Microbiology, 6(5), E8341( 2013).
-
Grammaticos P.C. And Diamantis A., Hellenic Journal Of Nuclear Medicine, 11 (1), 2 (2008).
-
Totaro P. And Rambaldini M., Interactive Cardiovascular And Thoracic Surgery 8, 153 (2009).
-
Shastri J.P.A., Rupani M.G., Jain R.L., The Journal Of The Textile Institute, 103 (11), 1234 (2012).
-
Zhang LZ, Yu JC, Yip HY, Li Q, Kwong KW, Xu AW, Wong PK., Langmuir, 19, 10372 (2003).
-
Shahrokh S., Hosseinkhani B., Emtiazi G. Bioprocessing And Biotechniques J, 4, 3, (2014).
-
Ahmad N., Alam M.K., Shehbaz A, Khan A., Mannan A., Hakim S.R., Bisht D., Owais M. (2005), J. Drug Target 13 (10), 555 (2005).
-
Matan N., Rimkeeree H., Mawson A.J., Chompreeda P., Haruthaithanasan V., Parker M., Int J Food Microbiol, 107 (2), 180 (2006)
-
Chen M. And Chen S. Process For Preparing Antibacterial Antimildew Polyacrylic Fibres and Its Filter Net for Air Conditioner, Patent Number CN 1355335 (2002).
-
Zuhuang J. Bactericidal nano-silver cloth and its making process and use. Patent number CN 1387700 (2003).
-
Lee H.J., Yeo S.Y., Jeong S.H., J Mat Sci, 38, 2199 (2003).
-
Arizona Science Center. Nanosilver Socks Demonstration. First Piloted At Triple Play Days, 2008 File:///C:/Users/Abed/Downloads/090415F5OP_Lib_Benntwetmorejand.Pdf (Accessed on April, 2014).
-
16 Raffi M., Hussain F., Bhatti T.M., Akhter J.I., Hameed A., Hasan M.M., Journal of Materials Science and Technology, 24 (2), 192 (2008).
-
Kamat P.V., Flumiani M., Hartland G.V., J Phys Chem, B102, 3123 (1998).
-
Kumar S.A., Kazemian M., Abyanehm.K., Gosavi S.W., Kulkarni S.K., Pasricha R., Ahmad A, Khan M.I., Biotechnology Letters, 29, 439 (2007).
-
Jain P. And Pradeep T., Biotechnology And Bioengineering, 90(1), 59 (2005).
-
Kirby-Bauer Antibiotic Testing, Http://En.Wikipedia.Org/Wiki/Kirby-Bauer_Antibiotic_Testing. Wikipedia CC BY-SA 3.0. (Accessed in April, 2014).
-
Sneath P., Mair N., Sharpe M., Holt J., Bergey's Manual Of Systematic Bacteriology, Vol. 2, Williams & Wilkins, Baltimore, Md, USA. (2000).
-
Mendes J.J., Marques A.C., Vilela C., Neves J., Candeias N., Cavaco S.P., Melo C.J., Diabetes Res. Clini. Pract., 95, 153 (2012)
-
Hong K.H., Park J.L., Sul, I.H., Youk J.H., Kang T.J., Journal Of Polymer Science, 44, 2468 (2006).
-
Sylvain B; Issenhuth-Jeanjean S, Grimont PAD., Journal of Clinical Microbiology, 42(8), 3388 (2004).
-
Gupta A.K. And Cooper E.A., Mycopathologia, 166, 353 (2008).
-
Deitch E.A., Marino A.A., Malakanok V., Albright J.A., the Journal of Trauma 27(3), 301 (1987).
-
Dibrov P., Dzioba J., Gosink K.K., Hặse C.C., Antimicrob Agents Chemother, 46, 2668 (2002).
-
Sondi I. and Salopek-Sondi B., Journal of Colloid And Interface Science, 275: 177 (2004).
-
Lansdown A.B.G., Curr Probl Dermatol. Basel, Karger, 33, 17 (2006).