Effects of Environmental Factors and Alien Plant Invasion on Native Floral Diversity in Mt. Manunggal, Cebu Island, Philippines
1
Research Institute for Tropical Biology and Pharmacological Biotechnology,
Cebu Normal University,
Osmeña Boulevard, Cebu City,
6000
Philippines
2
Biology Department, School of Sciences, College of Arts and Sciences,
Cebu Normal University,
Osmeña Boulevard, Cebu City,
6000
Philippines
3
Department of Biology and Environmental Science,
College of Science, University of the Philippines Cebu,
Cebu City,
6000
Philippines
Corresponding author Email: garcesjj@cnu.edu.ph
DOI: http://dx.doi.org/10.12944/CWE.13.3.12
Copy the following to cite this article:
Garces J. J. C, Flores M. J. L. Effects of Environmental Factors and Alien Plant Invasion on Native Floral Diversity in Mt. Manunggal, Cebu Island, Philippines. Curr World Environ 2018;13(3). DOI:http://dx.doi.org/10.12944/CWE.13.3.12
Copy the following to cite this URL:
Garces J. J. C, Flores M. J. L. Effects of Environmental Factors and Alien Plant Invasion on Native Floral Diversity in Mt. Manunggal, Cebu Island, Philippines. Curr World Environ 2018;13(3). Available from: http://cwejournal.org?p=1114/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2018-09-02 |
|---|---|
| Accepted: | 2018-12-13 |
| Reviewed by: | 
 Pervaiz A. Dar
Pervaiz A. Dar
|
| Second Review by: |

 Martin Hejda
Martin Hejda
|
| Final Approval by: | Dr. Mohammad Oves |
Introduction
Plant diversity patterns and composition can be influenced by several abiotic and biotic factors at different spatial scales. At local scales, plant diversity and composition are influenced by geophysical factors (i.e., soil, topography), land use, and biotic interactions. At regional scales, plant communities are influenced by climatic factors and biogeographical processes such as the action of pollinators and dispersal patterns.1 Islands nations like the Philippines tend to have high endemicity than countries in continental landmasses. However, these islands are also more vulnerable to biological invasions.2-4 Biological invasions affect biodiversity worldwide.5-6 As ecosystems are unceasingly degraded by anthropogenic activities, biological invasion studies became a mainstream discourse in improving our knowledge of propagule pressure, assembly time, and invasion patterns of invasive alien species (IAS), including the substantial progress in understanding the mechanisms of invasion processes.6-7
Invasive alien plants (IAPs) are species which can be introduced intentionally or unintentionally.2,8 In the Philippines, Sinohin and Cuaterno documented around 475 IAPs that have been introduced since the prehistoric times.9 The introduction of IAPs originated in Malayan-Polynesian terrestrial boundaries during the Spanish-American regime (via Acapulco trade) which may have included some common agricultural crops.2,10-11 When these IAPs are introduced intentionally, there could also be the unintentional introduction of detrimental species such as weeds, and pathogens in the soil.8,12 Suitable environmental factors and escalating anthropogenic pressures provide novel opportunities for IAPs to proliferate, leading to adverse changes in the soil dynamics, native floral composition, and other ecosystem processes.13-14 Where these IAPs proliferate, displacement, and in worst cases, extinction of native plants is observed as a result of nutrient competition among edaphic biota and introduction of new parasites and pathogens.8,12,15
In order to obtain more information on how native plant communities respond to environmental factors and the proliferation of IAPs, these objectives were carried out in the forested areas of Mt. Manunggal, Cebu Island, Philippines: (a) measure and compare the different environmental factors (altitude, light intensity, soil pH, soil temperature, rainfall and relative humidity) atinvaded plot (Site 1) and adjacent uninvaded plot (Site 2) sites of Mt. Manunggal, Cebu Island, Philippines; (b) determine the impacts of selected environmental factors to native and IAPs between Site 1 and Site 2 and (c) determine the impacts of IAPs on native floral species.
Materials and Methods
Study Area
Mount Manunggal is located on the steep mountainous region of Balamban, Cebu Island, Philippines.19 It has a total area of 500 hectares at 10°27'39.41 "N latitude and 123°46'50.72 "E longitude (Fig. 1). Since its announcement as part of the Central Cebu Protected Landscape (CCPL) under Republic Act 7586, the National Integrated Protected Area Systems (NIPAS) Act, Mt. Manunggal has undergone numerous changes and disturbances.16 Coolest temperature recorded was 17ËšC, and the hottest was 32ËšC; maximum annual rainfall peaked at 401.2 mm but averaging only 210.8 mm.17 The place is dominated by alternating hills, with soils that have tertiary basic igneous rocks and volcanic ashes.18 A gratuitous permit was secured from the Department of Environment and Natural Resources-Region VII (DENR-Region VII) before the conduct of the study.
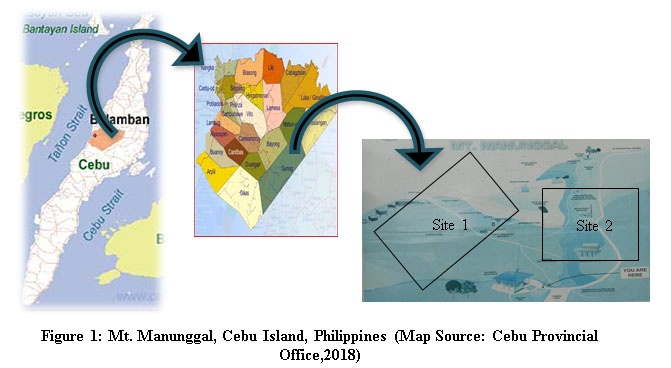 |
Figure 1: Mt. Manunggal, Cebu Island, Philippines (Map Source: Cebu Provincial Office, 2018) |
Site Selection and Sampling Site Establishment
A reconnaissance survey was conducted to observe the physical environment, plant species, and anthropogenic activities in Mt. Manunggal, Cebu Island, Philippines. Two (2) study sites (Site 1 and Site 2) were chosen based on comparable elevation, edaphic and climatic factors and the magnitude and intensity of anthropogenic activities.20 Site 1 (invaded plot) is a campsite with more anthropogenic activities occurring in the area. Site 2 (adjacent uninvaded plot) is found at the peak of the mountain, where anthropogenic activities are less rampant. A 100m x 50m area was established in each study site, where three (3) 100m-transect lines were positioned with a distance of 10m from each other. Four 20m x 20m quadrats in each transect were laid out alternately from left or right with a 20m interval to randomly cover each study site. A total of 24 quadrats from the six (6) transects were randomly selected for sampling.
Floral Assessment
A Descriptive Survey Design or a total count or census of all the plant (native and IAPs) population was employed using a Transect-Quadrat Method.21 The name of each plant species and the presence and the type of anthropogenic activities were identified per quadrat. Environmental parameters (altitude, soil pH, soil temperature and light intensity) were identified ‘in situ’ to determine its impacts to native and alien species abundance and diversity.
Nomenclature
There was no collection of alien and native plants performed at both study sites. Most of the plant species were identified in the field with the help of key informants/local villagers. Plants identified were confirmed based on standard taxonomic guide for native and alien plants: (1) International Union for Conservation of Nature (IUCN); (2) Asia-Pacific Forest Invasive Species Network (APFISN); (3) Global Invasive Species Database (GISD); and (4) Co’s Digital Flora database to validate the scientific names of the alien and native plant species present at the two study sites. Unidentified species were photographed for further identification at the Department of Environment and Natural Resources-Region VII (DENR-VII) by forestry experts.
Vegetation Analysis
To determine the taxonomic richness, evenness, and diversity of alien and native plant species, the following formula was used:
Species abundance = (Total # of species A/ Total # of all species) x 100 where: ln = the natural logarithm; S= the number of species encountered.
Species richness = no. of species found in each quadrat;
For species diversity, Shannon-Wiener Index (H’) was used22:
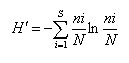
Where: ni= number of individuals; N= total number of species
To calculate Simpson’s Index of Diversity, the formula used was:
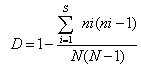
Where: ni= number of individuals; N= total number of species
To calculate evenness, the following were employed:
Hmax = and where: ln = the natural logarithm; S= the number of species encountered23
Æ‘= H/Hmax where: H= Shannon’s Index (Pielou, 1966)
Regression Analysis
To test the relationship between alien plant species abundance as predictors and native plant species abundance as the response variable, regression analysis was also employed, following the formula:
Y = b0 + b1X1 + b2X2…+ bqXq
where: Y= response variable; X1, X2… Xq = predictors; b0, b1, b2…bq, = partial regression coefficients of the predictors.
Furthermore, a t-test was used to measure and determine the significant difference of environmental parameters in both sites. Pearson correlation analysis was utilized to test the relationship between the environmental parameters of both sites. It was also employed to reflect the correlation between environmental to native and alien plant compositions. All data were subjected to Minitab Version 17.
Results and Discussions
Environmental Factors
Table 1 shows that except for soil pH, all other environmental factors such as altitude (t = -14.11, p=0.000), soil temperature (t=2.68, p=0.014), and light intensity (t = -2.61; p=0.016) significantly differed between study sites. Site 2 was located in a significantly higher elevation than Site 1. With an increase in elevation, soil temperature significantly decreased (r= -0.441, p=0.031), while soil pH and light intensity increased (r=0.859, p=0.038; r=0.480, p=0.017, respectively). These relationships could be attributed to the complex topography and geology of Mt. Manunggal, the variation in floral cover, and degree of anthropogenic activities (Table 3) in the study sites.
Floral Composition, Richness, Abundance, and Diversity
In terms of species richness, Site 1 has 62 plant species, made up of 37 (59.67%) IAPs and 25 (40.32%) native species. Meanwhile, Site 2 had 30 (54.55%) IAPs and 25 natives (45.45%) species for a total of 55 plant species. However, there were more IAP individuals in Site 2 at 32,504 (76.18%) compared with 10,165 (23.82%) in Site 1. Inversely, native plant abundance was higher in Site 1 at 354 individuals (60.10%) while Site 2 had 235 individuals (39.90%). The number of individuals per species within and across the study sites varied, with Site 1 having higher species richness but low abundance while Site 2 had the opposite. It is evident though that in both sites, species richness and abundance were higher for IAPs than the native plants. The stages of invasion by IAPs include (1) transport, (2) establishment, and (3) spread/invasion.6,25 In the first stage of invasion, anthropogenic activities (e.g. land tillage, trade, and tourism) determine the fate of introduction of IAPs in lower altitudes.6 Residence time and propagule pressure are factors in terms of introduction and establishment of IAPs in its newest environment. The longer the residence time of species in the region, the more propagules are gradually established in an area.6,26 This increases the probability of founding the new population in the site.6 An abundance of IAPs in Mt. Manunggal can be attributed to suitable environmental factors which are present at higher altitudes.26-27
Table 4 shows that Site 1 (H’= 2.76; D=0.10) had higher diversity than Site 2 (H’= 2.41; D=0.11). However, it seems that IAPs were the ones contributing much to the diversity in both sites, although Site 1 had higher IAP richness of 37 compared with 30 in Site 2. Since there were more disturbances at Site 1 brought about by anthropogenic activities, it is not surprising to have a higher alien plant species richness in this site. The chance of having more vectors (i.e. humans, animals, etc.) at Site 1 that intentionally or unintentionally introduced these species of plants to establish, grow and proliferate is higher. Anthropogenic activities in Table 3, which has brought social and economic benefits to many people, can contribute to an increased abundance of IAPs, thus facilitating ‘invasional meltdown’ in the whole ecosystem.28 Shannon diversity index (H’) measures the degree of uncertainty in a sample area. If the diversity is low, the certainty of picking a particular species at random is high. If it is high, it is then difficult to predict the identity of a randomly picked individual. This implies that the higher the resulting value, the more diverse the site is; high diversity means high uncertainty. H’ includes both species richness and species evenness. Therefore, it allows knowing not only the number of species but how the abundance of the species is distributed among all the species in the community. Site 2 had a higher Pielou’sevenness (PE) index of (PE=0.73) compared with (PE=0.67) for Site 1 (Table 4). A dominance of IAPs against native plants in all study sites of Mt. Manunggal was evident in this study. As a secondary forest, most of the plants were introduced by the government for reforestation, thereby facilitating introduction and establishment of IAPs.29-30 The Philippine government, through the Department of Environment and Natural Resources (DENR), has intensified tree planting in most forest ecosystems. However, most of the trees planted are alien trees (e.g. Swietenia macrophylla) for reforestation purposes, while Theobroma cacao (chocolate) as source of livelihood among forest settlers. Anthropogenic activities (i.e., farming, tilling of land, livestock, and poultry) hastened the establishment, growth, and proliferation of alien plants in the study sites. The continuous increase in the number of IAPs has potentially altered forest community structure and function, thereby threatening ecological and environmental significance of Mt. Manunggal. This could eventually lead to stochastic extinction of native species in the area.
Simpson’s diversity index (D) is employed to identify the probability of randomly sampled individuals belong to the same species or any categories other than species. The resulting value shows that the number nearest to 0 is infinitely diverse and the value nearest to 1 is less diverse. In this case, without taking into account the species richness, Site 1 (D=0.10) is more diverse compared to Site 2 (D=0.11), i.e. alien plants were concentrated and dominant at Site 1, composed of 37 alien plants compared to 30 alien plants at Site 2.
The observed distribution of both IAPs and native plants with respect to altitude vary depending on their biogeographical origin and environmental tolerance, as presented in similar investigations on islands worldwide.31-32 Studies have confirmed that species abundance, richness, and diversity respond to altitudinal gradients.33-34 The abrupt decrease or increase of IAPs and native plant occurrence along the gradient confirmed that altitude is a key factor in facilitating or hindering invasive alien invasion.35 Arteaga et al., showed that tropical and subtropical plant species are always more abundant along increasing altitude.36 Altitudinal distribution of both native and alien plant species is largely related to environmental stress gradients, dependent on altitude.37 The success and impacts of alien species also depend on their biological attributes, the environmental characteristics of the ecosystem and the biotic interactions with the receptive community.26 Alexander et al., confirmed that most alien plant species first arrive at low altitudes, where anthropogenic propagule pressure is greatest, and spread upward from there, either naturally or through human-mediated activities.38 The progressive addition of species, where the species found at high altitudes are those with the widest ranges that also occurred at low elevations.39
Table 1: The mean SE (minimum and maximum values in parentheses) and significant difference (t-test and p-value) of environmental (climatic and edaphic) parameters per site of Mt. Manunggal, Cebu Island, Philippines.
|
|
Site 1 |
Site 2 |
Site 1 and 2 |
|
||||||||||||||||
|
Environmental Parameters |
Alien |
Native |
Alien |
Native |
Alien |
Native |
|
|||||||||||||
|
r |
Significance |
r |
Signifi cance |
r |
Signifi cance |
r |
Signifi cance |
r |
Signifi cance |
r |
Significance |
|||||||||
|
Altitude |
-- |
-- |
-0.571* |
0.052 |
0.756** |
0.004 |
0.587* |
0.045 |
0.709 *** |
0.000 |
-0.421* |
0.040 |
||||||||
|
Soil temperature |
-- |
-- |
-- |
-- |
0.985** |
0.006 |
-- |
-- |
-- |
-- |
-- |
-- |
||||||||
|
Soil pH |
-- |
-- |
-0.679* |
0.015 |
-0.671* |
0.017 |
-0.605* |
0.037 |
-0.428* |
0.037 |
-0.579** |
0.003 |
||||||||
|
Light Intensity |
-- |
-- |
-- |
-- |
-0.795** |
0.002 |
-- |
-- |
-- |
-- |
-- |
-- |
||||||||
Remarks: *** Significant difference at the 0.001 level (2-tailed) ** Significant difference at the 0.01 level (2-tailed)
*Significant difference at the 0.05 level (2-tailed) --No significant difference
Table 2: Correlation coefficients of the selected environmental parameters and floral components of Site 1 and Site 2, Mt. Manunggal, Cebu Island, Philippines, October-November 2016.
|
Parameter |
Site 1 |
Site 2 |
t-test |
p-value |
|
Altitude (masl) |
927.26 ± 1.75 (831.00-998.00) |
976. 53 ± 1.41 (859.00-1050.00) |
-14.11*** |
0.000 |
|
Soil temperature (ºC) |
24.40 ± 0.37 (22.67-37.55) |
20.77 ± 0.28 (18.24-33.00) |
2.68* |
0.014 |
|
Soil pH |
3.39 ± 0.05 (2.15-5.35) |
3.37± 0.05 (2.42-5.10) |
-- |
-- |
|
Light Intensity (lux) |
526.20 ± 12.90 (205.0-989.00) |
686.20 ± 16.50 (88.30-1050.00) |
-2.61* |
0.016 |
Remarks: *** Significant difference at the 0.001 level (2-tailed) ** Significant difference at the 0.01 level (2-tailed).
*Significant difference at the 0.05 level (2-tailed) --No significant difference.
Table 3: Anthropogenic activities recorded at Site 1 and Site 2, Mt. Manunggal, Cebu Island, Philippines.
|
Anthropogenic Activities |
SITE 1 |
SITE 2 |
|
Animal grazing |
32 |
4 |
|
Hunting/poaching |
25 |
5 |
|
Livestock |
25 |
8 |
|
Farming |
45 |
11 |
|
Medicinal plants |
29 |
9 |
|
Abaca processing |
21 |
9 |
|
Carpentry |
22 |
3 |
|
Wood charcoal |
26 |
3 |
|
Slashing, cutting and burning |
21 |
4 |
|
Mountain climbing |
10 |
9 |
|
Camping |
10 |
7 |
Remarks: *** Correlation is significant at the 0.001 level (2-tailed) **Correlation is significant at 0.01 level (2-tailed).
* Correlation is significant at the 0.05 level (2-tailed) --Correlation is not significant.
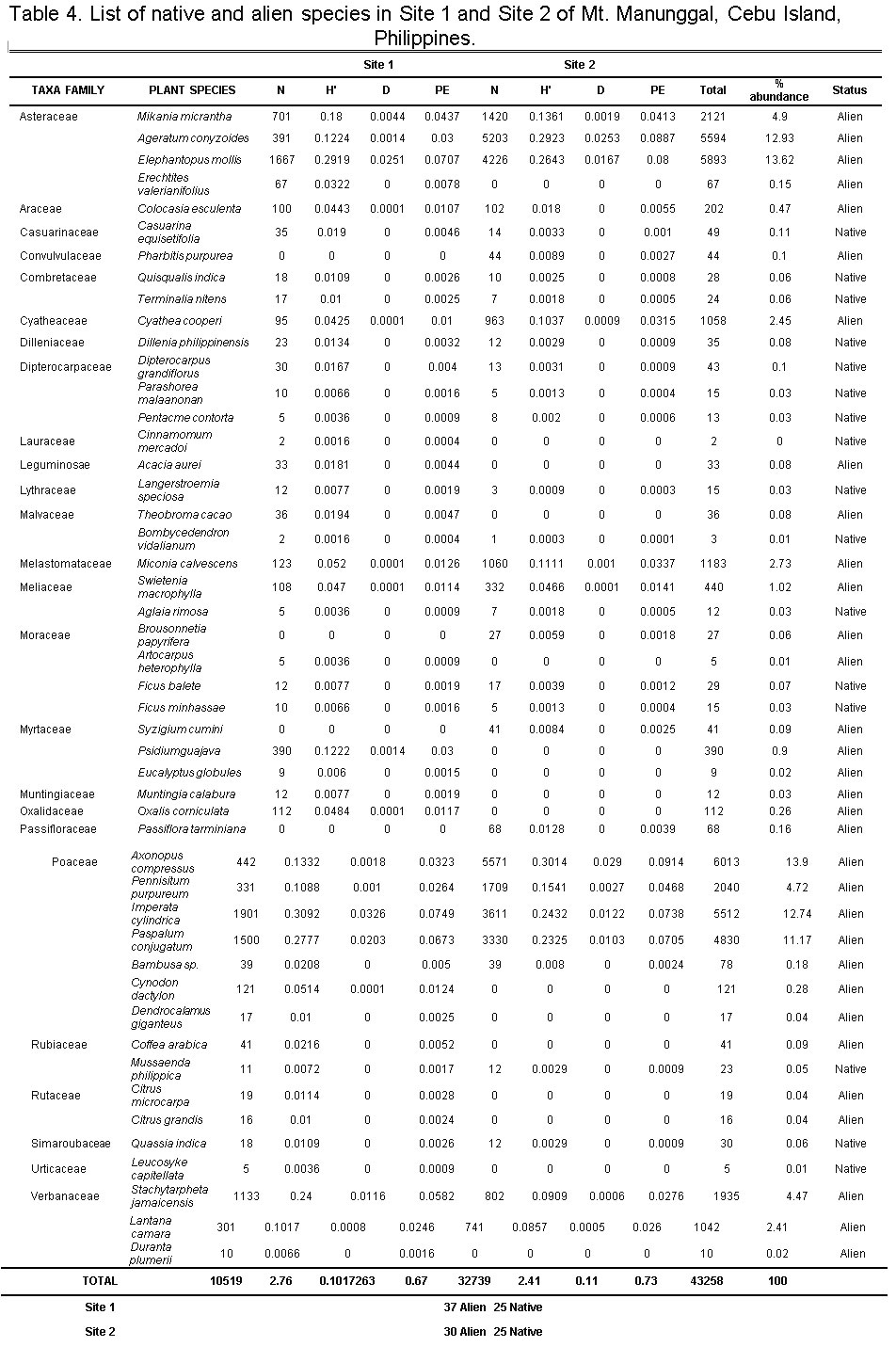 |
Table 4: List of native and alien species at Site 1 and Site 2 of Mt. Manunggal, Cebu Island, Philippines. Click here to view table |
Remarks: N-species abundance; D-Simpson diversity index
H’-Shannon-Weiner diversity index; PE-Pielou’s evenness
Remarks: N-species abundance; D-Simpson diversity index
H’-Shannon-Weiner diversity index; PE-Pielou’s evenness
Table 5: Pearson's correlation coefficients between environmental parameters and the floral component of Site 1 and Site 2 at Mt. Manunggal, Cebu Island, Philippines.
|
|
Site 1 |
Site 2 |
Site 1 and 2 |
|
||||||||||||||||
|
Environmental Parameters |
Alien |
Native |
Alien |
Native |
Alien |
Native |
|
|||||||||||||
|
r |
Significance |
r |
Signifi cance |
r |
Signifi cance |
r |
Signifi cance |
r |
Signifi cance |
r |
Significance |
|||||||||
|
Altitude |
-- |
-- |
-0.571* |
0.052 |
0.756** |
0.004 |
0.587* |
0.045 |
0.709 *** |
0.000 |
-0.421* |
0.040 |
||||||||
|
Soil temperature |
-- |
-- |
-- |
-- |
0.985** |
0.006 |
-- |
-- |
-- |
-- |
-- |
-- |
||||||||
|
Soil pH |
-- |
-- |
-0.679* |
0.015 |
-0.671* |
0.017 |
-0.605* |
0.037 |
-0.428* |
0.037 |
-0.579** |
0.003 |
||||||||
|
Light Intensity |
-- |
-- |
-- |
-- |
-0.795** |
0.002 |
-- |
-- |
-- |
-- |
-- |
-- |
||||||||
Remarks: *** Correlation is significant at the 0.001 level (2-tailed) **Correlation is significant at 0.01 level (2-tailed).
*Correlation is significant at the 0.05 level (2-tailed) --Correlation is not significant.
Results in Table 5 shows that with an increase in elevation, soil temperature significantly decreased (r=0.441, p=0.031), while soil pH and light intensity increased (r=0.859; p=0.038; r=0.480; p=0.017, respectively). The higher richness of IAPs in Site 1 could be because the majority of IAPs are best suited to soils with warmer temperature to allow seed germination and seedling emergence,41-42 pollination40 and vegetative development (node and leaf appearance rate), root development, node formation, and leaf surface formation.43 Soil pH did not significantly differ in both sites. Soils were generally acidic which resulted in a negative correlation with native and alien plant species in both sites (r=-0.428; 0.037; r=-0.579, p=0.003) (Table 1). Tropical forests soils are generally thin and acidic.18,44 These relationships could be attributed to the complex topography and geology of Mt. Manunggal, and the variation in floral cover, and degree of anthropogenic activity at both study sites.
The significant negative correlation of light intensity to IAP abundance of Table 5 at Site 2 (r= -0.795, p=0.002) was expected since it is located at higher altitude. Depending on the size and type of plant, the amount of available moisture, the relative humidity, and a myriad of other modifying factors, full exposure to the sun can damage plants.45-46 Physiologically, too much light would result in disturbed photosynthetic process, cellular respiration and stress on its biological and physiological rhythms which decreases the IAPs in forest ecosystems.47 Plant’s vegetative structure can also be affected, specifically in lesser root density, stunted stem growth, and arrest in fruit and seed formation.48 In other cases, some of the IAPs appear to be better suited than native plant species or vice versa in capturing and utilizing light, particularly in high light environments, and such as those characterized by relatively high levels of disturbance.49 It could be implied that the growth rate of invaders is condition- or context-dependent.50 This could be the main reason why some of the native plants and IAPs appear to be negatively correlated with light intensity. The universal growth-rate advantage of either native or alien plant species should be examined in longer growing condition.47-50
Table 6: Regression analysis of native and alien plant species composition of Site 1 in Mt. Manunggal, Cebu Island, Philippines.
|
Analysis of Variance |
|||||||||
|
Source |
DF |
Adj SS |
Adj MS |
F-Value |
P-Value |
||||
|
Regression |
1 |
1392 |
1392.5 |
11.05 |
0.009 |
||||
|
Alien_Abun |
1 |
1392 |
1392.5 |
11.05 |
0.009 |
||||
|
Error |
10 |
1253 |
123.4 |
||||||
|
Total |
11 |
2640 |
|||||||
|
Model Summary |
|||
|
S |
R-sq |
R-sq(adj) |
R-sq(pred) |
|
11.2037 |
52.4% |
47.75% |
21.43% |
Legend: p-value<α=0.05 – Significant at α=0.05 p-value>α=0.05 – Not Significant at α=0.05.
Table 7: Regression analysis of native and alien plant species composition of Site 2 in Mt. Manunggal, Cebu Island, Philippines.
|
S |
R-sq |
R-sq(adj) |
R-sq(pred) |
|
11.3011 |
49.18% |
44.21% |
19.34% |
Table 8: Analysis of coefficients for native and alien plant species composition of Site 1 in Mt. Manunggal, Cebu Island, Philippines.
|
Coefficients |
||||||||
|
Term |
Coef |
|
SE Coef |
|
T-Value |
|
P-Value |
VIF |
|
Constant |
|
3.00 |
|
9.07 |
0.33 |
|
0.74 |
|
|
Alien_Abun |
|
0.0331 |
0.0104 |
3.3 |
0.009 |
1.00 |
|
|
Legend: p-value<α=0.05 – Significant at α=0.05 p-value>α=0.05 – Not Significant at α=0.05.
Table 9: Analysis of coefficients for native and alien plant species composition of Site 2 in Mt. Manunggal, Cebu Island, Philippines.
|
Coefficients |
||||||||||
|
Term |
Coef |
SE Coef |
T-Value |
P-Value |
VIF |
|||||
|
Constant |
3.21 |
8.87 |
0.41 |
0.698 |
||||||
|
Alien_Abun_1 |
0.0348 |
0.0097 |
2.91 |
|
0.015 |
1.00 |
||||
Alien plant invasion could have caused a significant impact on native plant abundance in Site 1 (F(1,10)=11.05, p= 0.009, R2 = 52.48%, R2adjusted = 47.75%) and (Coef=0.0331; SE Coef= 0.0104; t=3.30; p=0.009; VIF=1.00). Similar impact was also observed in Site 2 based on the results presented in Table 7 and Table 9 where F (1,10)=9.67, p= 0.015, R2 = 49.18%, R2adjusted = 44.21%) and (Coef=0.0.0348; SE Coef= 0.0097; t=2.91; p=0.015; VIF=1.00). Studies of past introduction demonstrate that the effects of IAPs are complex and can permanently alter the structure of communities.51 IAPs pose a threat to native plant communities globally, especially where these communities are disturbed.52 Site 1 serves as the point of entryway for the introduction and establishment of alien plant species.53 In animal domestication, animals consume grasses and fruits and even act mainly as seed dispersers, which assist in pollen and seed dispersal.54 Kolb et al., also justified ‘abaka’-making and fencing as effective transporters of IAPs in sites which are highly-disturbed by animals and humans.55 For example, species of Axonopus compressus, Elephantopus mollis, and Ageratum conyzoides seemed to be the top IAPs at a global scale as shown in the study by Dogra et al.,.54 These herbaceous plants are also stoloniferous and runners which rapidly spread out in the area once it is introduced and finally adapted with the environment.56 These plants also possess dense masses of broad-leafed seedlings which can grow in areas with sufficient moisture, coupled with warm temperature and moderate light exposure.6,39,49-50 These IAPs are responsible for extensive and unpredictable irreversible changes to the natural habitats in different continents worldwide.57 For example, L. camara, which originated in Tropical America, has become common throughout the country in the forests, plantations, agricultural land, disturbed areas, grasslands, and wetlands, riparian and urban areas.58
Conclusions and Recommendations
It is clear that invasive alien plants and environmental factors (i.e. altitude, soil temperature, and light) may have negative, positive, or neutral effects on the population success of native plant species. These results could be attributed to the complex topography and geology of Mt. Manunggal, and the variation in floral cover, and degree of anthropogenic activities at both study sites. The vegetation survey revealed domination of alien plants in terms of species richness and abundance at both sites. At Site 2, both alien and native plant abundance responded positively to elevation, and negatively to soil pH and light intensity. At Site 1, only native plant abundance responded negatively to elevation and soil pH. High invasion rate at Site 1 and Site 2 could be attributed to both rampant anthropogenic activities and favorable environmental conditions. If neglected, these alien plants would continue to proliferate in Mt. Manunggal and further threaten the native plants in the forest and grasslands, as well as in nearby areas. Conduct of regular assessments of the negative impacts by alien plants to native floral diversity, enhanced by anthropogenic activities, must, therefore, serve as bases in future directions and implication for restoration and conservation of the remaining forests of Mt. Manunggal, Cebu Island, Philippines. Sustainable biodiversity conservation programs must also be prioritized and conservation biologists, farmers, local villagers, government, and landowners should come together in the planning, implementation, and monitoring of these programs.
Acknowledgments
We would also like to thank Prof. Judith R. Silapan and Dr. Patricia Anne G. Nazareno of the University of the Philippines Cebu and Mr. William Navares of DENR-VII for their valuable comments and suggestions. Financial support for this work, which is part of an M.Sc. thesis, was provided by the Philippine Commission of Higher Education (CHED) and the Department of Environment and Natural Resources Region 7 (DENR-VII). This work was also supported by Research Institute for Tropical Biology and Pharmacological Biotechnology (RITBPB) of Cebu Normal University. Finally, we would like to thank the anonymous referees who gave important comments and suggestion for the improvement of this paper.
References
- Paudel S., Benavides J. C., Macdonald B., Longcore T., Wilson G. W. T., Loss S. R. Determinants of native and non-native plant community structure on an oceanic island. Ecosphere. 2017;8(9): e01927. doi: 10.1002/ecs2. 1927.
- Elton CS. The ecology of invasion by animals and plants. Menthuen, London. 1958.
- Vitousek PM, Antonio CM, Loope LL, Westbrooks R. Biological invasions as global environmental change. American Scientist. 1996;84(5):468.
- Lenda M, Witek, Skorka P, Moron D, Woyciechowski M. Invasive alien plants affect grassland ant communities, colony size, and foraging behavior. Biol Invasions. 2013;15:2403-2414 doi. 10-1007/s10530-013-0461-8.
- Didham R. K., Tylianakis J. M., Hutchison M. A., Ewers R. M., Gemmell N. J. Are invasive species the drivers of ecological change? Trends in Ecology & Evolution. 2005;20(9):470-474.
CrossRef - Pyšek P, Jarošík V, Pergl J. Alien plants introduced by different pathways differ in invasion success: unintentional introductions as a threat to natural areas. PLoS One. 2011;6(9):e24890.
- Pyšek P., Richardson D. M. Invasive species, environmental change and management, and health. Annual Review of Environment and Resources. 2010;35:25-55.
CrossRef - Convention on Biological Diversity (CBD). Retrieved from: https://www.cbd.int/ doc/legal/cbd-en.pdf.2006.
- Sinohin V, Cuaterno W. Invasive Alien Species Resource Directory for the Philippines. [Internet] Retrieved from: http://www.arcbc.org.ph/arcbcweb/pdf/ vol2no4/ 30-32_invasive_philippines.pdf. 2002.
- di Castri F. History of biological invasion with emphasis on the Old Word. Pages 1-30. In J. Drake, F., di Castri, R. Groves, F. Kruger, H.A. Mooney, M. Rejmanek, and Williamson, M. (eds.). Biological invasion: a global perspective. New York, USA: Wiley. 1989.
- Crawley M. J., Harvey P. H., Purvis A. Comparative ecology of the native and alien floras of the British Isles. Philosophical Transactions of the Royal Society of London Series B- Biological Sciences. 1996;351:1251-1259.
CrossRef - Ecological Society of America (ESA). Invasive Species. [Internet] Retrieved from: http://www.esa.org/education/edupdfs/invasion.pdf.2004.
- Tallamy D. W. Do alien plants reduce insect biomass? Conservation Biology. 2004;18(6):1689-1692.
CrossRef - Vega-Ross M. Effect of invasive plants on herbivores and herbivory preferences. [Internet] Retrieved from: https://underc.nd.edu/assets/174545/fullsize/ vega_ross_underc_w_2013.pdf. 2013.
- United Nations Environmental Programme (UNEP). [Internet] Retrieved from: http://www.unep.org/yearbook/2012/. 2012.
- Department of Environment and Natural Resources (DENR). Ecosystem Research and Development Board Annual Report. Retrieved from: [Internet] http://erdb.denr.gov.ph/files/erdb_ar09.pdf. 2013.
- Philippine Atmospheric, Geophysical, Astronomical Services Administration (PAGASA) 2016. Annual Report. Retrieved from: https://pubfiles.pagasa.dost.gov.ph/ppds/Annex82.pdf. 2016.
- Department of Agriculture (DA). Annual Report on Agriculture. Retrieved from: http://agricoop.nic.in/sites/default/files/Annual_rpt_201617_E.pdf. 2016.
- Cebu Provincial Map. Retrieved from: https://www.cebu.gov.ph/about-cebu/map-of-cebu/. 2018.
- Perner J., Audorff V. Effects of plant diversity, community composition and environmental parameters on productivity in montane European grasslands. PubMed. Oecologia. 2005;142:606-615. DOI. 10.1007/s00442-004-1749-2.
CrossRef - Braun-Blanquet. Plant Sociology. New York.1932.
- Shannon C. E., Wiener W. The Mathematical Theory of Communication. University of Illinois Press: Ur-bana, Illinois. 1963.
- Heip, Carlo. A new index measuring evenness. Journal of the Marine Biological Association of the United Kingdom. 1974;54(3):555-557.
CrossRef - Pielou E. C. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology. 1966;13:131-44.
CrossRef - Rejmanek M., Richardson D. M., Higgins S. I., Pitcairn M. J., Grotkopp E. Ecology of invasive plants: state of the art. Scope-Scientific Committee on Problems of the Environment International Council of Scientific Unions. 2005;63:104.
- Vilà M., Basnou C., Pyšek P., Josefsson M., Genovesi P., Gollasch S., Hulme P. E. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Frontiers in Ecology and the Environment. 2004;8(3):135-144.
CrossRef - Sax D. F., Kinlan B. P., Smith K. F. A conceptual framework for comparing species assemblages in native and exotic habitats. Oikos. 2005;108(3):457-464.
CrossRef - Simberloff D., Schmitz D. C., Brown T. C. Strangers in paradise: impact and management of nonindigenous species in Florida. Island press.1999.
- Department of Environment and Natural Resources (DENR). Ecosystem Research and Development Board Annual Report. Retrieved from: [Internet] http://erdb.denr.gov.ph/files/erdb_ar09.pdf.1995
- National Report to the Convention on Biological Diversity (NRCBD). Canada’s 4th National Report to the United Nations Convention on Biological Diversity. [Internet] Retrieved from https://www.cbd.int/doc/world/ca/ca-nr-04-en.pdf. 2013.
- Wilson E. O. The little things that run the world (the importance and conservation of invertebrates). Conservation Biology. 1987;1:344-346.
CrossRef - Falk D. A., Palmer M. A., Zedler J. B. Foundations of restoration ecology. Washington, DC: Island Press. 2006;14-41.
- Perner J., Wytrykush C., Kahmen A., Buchmann N., Egerer I., Creutzburg S., Odat N, Aurdoff., Weisser W. W. Effects of plant diversity, plant productivity and habitat parameters on arthropod abundance in montane European grasslands. Ecography. 2005;28:429-442. ISSN 0906-7590.
- Bartomeus I., Vilà M., Santamaría L. Contrasting effects of invasive plants in plant-pollinator networks. Oecologia. 2008;155(4):761-770.
​​​​​​​CrossRef - Borer E. T., Seabloom E. W., Shurin J. B., Anderson K. E., Blanchette C. A., Broitman B., Halpern B. S. What determines the strength of a trophic cascade? Ecology. 2005;86(2):528-537.
​​​​​​​CrossRef - Albrecht M., Duelli P., Schmid B., Müller C. B. Interaction diversity within quantified insect food webs in restored and adjacent intensively managed meadows. Journal of Animal Ecology. 2007;76(5):1015-1025.
​​​​​​​CrossRef - Gratani L. Plant phenotypic plasticity in response to environmental factors. Hindawi Publishing Corporation. Advances in Botany. 2014. Article ID 208747. Retrieved from: http://dx.doi.org/10.1155/2014/208747.​​​​​​​
CrossRef - Alexander J. M., Kueffer C., Daehler C. C., Edwards P. J., Pauchard A., Seipel T. MIREN Consortium. Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc Natl Acad Sci USA. 2011;108:656–661.
​​​​​​​CrossRef - Pyšek P., KÅ™ivánek M., Jarošík V. Planting intensity, residence time, and species traits determine invasion success of alien woody species. Ecology. 2009;90(10):2734-2744.
​​​​​​​CrossRef - Hatfield K. J., Boote B. A., Ziska L. H., Izaurralde R. C., Ort A. M., Wolfe D. W. Climate Impacts on agriculture: implication for crop production. Agron J. 2011;103:351-370.
- Sato S. The effects of moderately elevated temperature stress due to global warming on the yield and the male reproductive development of tomato. Hort Research. 2006;60:85-89.
- Kadir G. S., Al-Khatib K. Strawberry growth and productivity as affected by temperature. HortScience. 2006;41:1423-1430.
- Hatfield J. L., Boote K. J., Fay P., Hahn L., Izaurralde C., Kimball B. A., Mader T, Morgan J., Ort D., Polley W., Thomson A., Wolfe D. The effects of climate change on agriculture, land resources, water resources, and biodiversity in the United States (2008). Agron J. 2008;68.
​​​​​​​CrossRef - F. A. O. The State of Food Insecurity in the World 2016. Meeting the 2016 international hunger targets: taking stock of uneven progress. Food and Agriculture Organization Publications, Rome. 2016.
- Yamashita N., Ishida A., Kushima H., Tanaka N. Acclimation to suddenly increased light favoring an invasive over native trees in subtropical islands, Japan. Oecologica. 2000;125:412-419.
​​​​​​​CrossRef - Wu Y. Y., Liu C. Q., Li P. P., Wang J. Z., Xing D., Wang B. L. Photosynthetic characteristics involved in adaptability to Karst soil and alien invasion of paper mulberry (Broussonetia papyrifera L. Vent.) in comparison with mulberry (Morus alba L.). Photosynthetica. 2009;47(1):155-160.
​​​​​​​CrossRef - Tyagi A., Yadav A., Mani A., Sribash R. High light intensity plays a major role in the emergence of population-level variation in Arabidopsis thaliana along an altitudinal gradient. Scientific Reports. 2006;6:26160. doi:10.1038/srep26160.
​​​​​​​CrossRef - Yokawa K., Fasano R., Kagenishi T., Baluška F. Light as stress factor to plant roots– a case of root halotropism. Frontiers in Plant Science. 2014;5:718.
​​​​​​​CrossRef - Proulx S. R., Promislow D. E., Phillips P. C.Network thinking in ecology and evolution. Trends in Ecology & Evolution. 2005;20(6):345-353.
​​​​​​​CrossRef - Pyšek P., Blackburn T. M., García-Berthou E., Perglová I., Rabitsch W. Displacement and local extinction of native and endemic species. In Impact of biological invasions on ecosystem services. Springer International Publishing. 2017;157-175.
​​​​​​​CrossRef - Carlton J. T. Community assemblage and historical biogeography in the North Atlantic Ocean: The potential role of human-mediated dispersal vectors. Hydrobiol. 2003;503:1-8.
​​​​​​​CrossRef - D’Antonio C. M., Levine J. M., Thomson M. Ecosystem resistance to invasion and the role of propagule supply: A California perspective. J. Mediterr. Ecol. 2001;27:233-245.
- Fridley J. D., Stachowicz J. J., Naeem S., Sax D. F., Seabloom E. W., Smith M. D., Holle B. V. The invasion paradox: reconciling pattern and process in species invasions. Ecology. 2007;88(1):3-17.
​​​​​​​CrossRef - Dogra K. S., Sood S. K., Dobhal P. K., Sharma S. Alien plant invasion and their impact on indigenous species diversity at a global scale: A review. Journal of Ecology and Natural Environment. 2010;2(9):175-188. ISSN 2006- 9847. Academic Journals.
- Kolb A., Alpert P., Enters D., Holzapfel C. Patterns of invasion within a grassland community. J Ecol. 2002; 90:871-881.
​​​​​​​CrossRef - Zhang J. T., Dong Y. Factors affecting species diversity of plant communities and the restoration process in the loess area of China. Ecological Engineering. 2010;36:345-350.
​​​​​​​CrossRef - Heywood V. H., Iriondo J. M. Plant conservation: old problems, new perspectives. Biological conservation. 2003;113(3):321-335.
CrossRef - National Focal Point for APFISN. India, Stocktaking of National Forest Invasive Species Activities, (India Country Report 101005). Delhi: Ministry of Environment and Forests. 2015.







