Coconut Shell: A carrier for the removal of lead (II) from aqueous solution
U.E. Chaudhari *
1
Department of Chemistry,
S.R.R. Lahoti,
Science College,
Morshi,
India
DOI: http://dx.doi.org/10.12944/CWE.2.1.10
The studies on removal of Lead (II) were conducted using Coconut shell. Adsorption efficiency has been evaluated. The effect of pH, contact time, adsorbent dose, concentration of metal, particle size and temperature were studied. The result reveals that Langmuir and Freundlich isotherms are followed during adsorption process. Thermodynamics parameter indicates the feasibility of the process. Kinetic studies have been performed to understand the mechanism of adsorption. Column studies have been carried out to compare these with batch capacities.
Copy the following to cite this article:
Chaudhari U.E. Coconut Shell: A carrier for the removal of lead (II) from aqueous solution. Curr World Environ 2007;2(1):51-55 DOI:http://dx.doi.org/10.12944/CWE.2.1.10
Copy the following to cite this URL:
Chaudhari U.E. Coconut Shell: A carrier for the removal of lead (II) from aqueous solution. Curr World Environ 2007;2(1):51-55. Available from: http://www.cwejournal.org/?p=629
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2007-03-06 |
|---|---|
| Accepted: | 2007-04-13 |
Introduction
The Twentieth century started with an extensive damage to the natural resources.1 Unplanned industrialization, urbanization, pollution explosion, change in life-style, over exploitation of natural resources, commercial establishment and modern agriculture practices have degraded the quality of environment.
The main effects being faced are:
- Continental invasion of air and water.
- Marine pollution through waste discharges.
- Release of variety of chemical and biological contaminants into the water bodies, on land and in air.
- Ground water pollution.
- Acid rains and nuclear fallout.
These effects are not only covering the pollution of environment but also are responsible in creating genetic erosion in plants, animals including human beings and microorganisms. Water is a prime natural resource and is a basic human need. The availability of adequate water supply in terms of its quality and quality is essential for the existence of life.
Water is available in nature as surface water and ground water through the self-purification mechanisms like physical, chemical and microbiological processes an natural bodies, are carried out in nature. However, natural water is rarely suitable for direct consumption to human beings. Rapid industrialization and population growth resulted to generation of large quantities of wastewater and causing problem of their disposal. Industrial waste constitutes the major source of various kinds of metal pollution in natural water. The presence of heavy metals in the environment has been of great concern because of their increased discharge, toxic nature and other adverse effects on the receiving streams. When the concentration of toxic metal ions exceed tolerance limit, they may become real health concern2. There is an immediate need to introduce cleaner technologies to minimize the pollution and to protect the degrading environment. It is not possible to achieve zero waste discharge, but it is an essential to treat the waste.
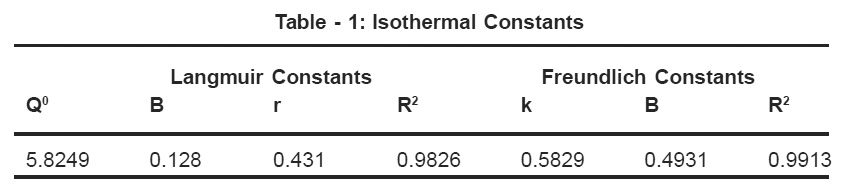 |
Table - 1: Isothermal Constants Click here to view table |
Among the toxic heavy metal ion, which present in potential health hazard to aquatic animals and human like Pb, Cd, Cr, V, Bi and Mn are important.
The maximum to tolerance limit for Lead II for public water supply are 0.1mg/L. Toxicity of metal depends on the type of metal, does and the ionic form. Lead is extensively used in printing, manufacture of paints, water pipes, storage battery manufacture, pottery and soldering operation etc. Besides it is used as a antiknock agent in gasoline. Toxicity of Lead3 includes anemia poisoning like acute abdominal colic and syndrome of acute encephalopathy. It cause mental deterioration convulsive seizures, serve central nervous system depression and death.
Results and Discussion
Literature survey reveals that, there are many methods namely coagulation, precipitation, ion exchange and adsorption, for removal of Lead II ​​metal ions from aqueous medium. However, adsorption is an easy and economical process for removal and retrieval of caption from aqueous medium. Efficiency of adsorption process mainly depends on nature or adsorbent, adsorbate, pH, concentration, temperature, time of agitation etc.
These cheap and efficient adsorbents can carry to cater to need of population in the rural areas and the population in the industrial area where safe drinking water is not available. In the present study, Lead (II) is removed by using coconut shell4-9 as a adsorbent.
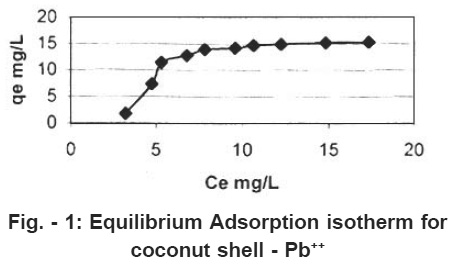 |
Figure - 1: Equilibrium Adsorption isotherm for coconut shell - Pb++ Click here to view figure |
Adsorbent
The coconut shell was first dried at a temperature of 160°C for 6 hours. After grinding it was sieved to obtain average particle size of 200 mesh. It was then washed several times with distilled water to remove dust and other impurities. Finally it was dried again in an over at 50°C for 6 hours. The adsorbent was then stored in desiccators for final studies.
Batch Study
The dried amount of 0.5gms of coconut shell was taken in 250ml reagent bottle any synthetic solution 200ml containing various concentration of Lead (II) ion was added and system is equilibrated by shaking the contents of the flasks at room temperature so that adequate time of contact between adsorbent and final concentration of metal ion. Lead (II) was determined by spectro-photometry10 using Dithizone method at 515nm against a reagent blank. The spectrophotometer, systronic (model 104) was used to measure the concentration of Lead (II) ions.
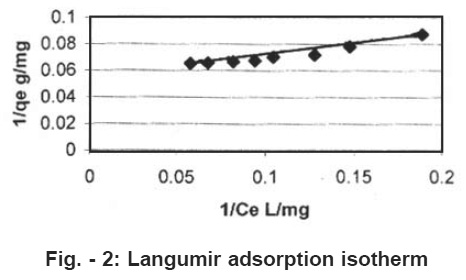 |
Figure - 2: Langumir adsorption isotherm Click here to view figure |
Equilibrium adsorption isotherm for Ce verses qe, plotted for coconut shell are shown in Figure1. The adsorption capacity in mg/L was calculated then the equation.
qe = (Co-Ce) V/M
where, Co is the initial concentration of Lead (II)
Ce is concentration of Lead (II)
at equilibrium in mg/L
V is the volume of solution in litre and
M is the mass of adsorbent in grams
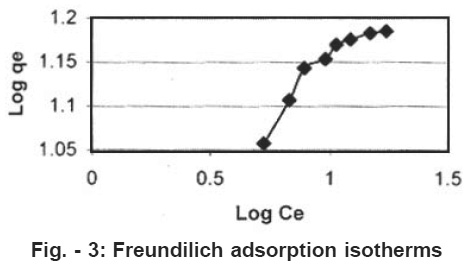 |
Figure - 3: Freundilich adsorption isotherms Click here to view figure |
Adsorption Isotherms
Equilibrium isotherms was studied for both Langmuir and Freundlich isotherms. The results are shown in figure 2 and 3, which illustrate the plot of Langmuir and Freundlich isotherms of coconut shell for Lead (II). The saturated monolayer can be represented by:
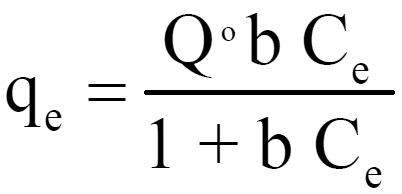
The lineraised form of the Langmuir isotherms is
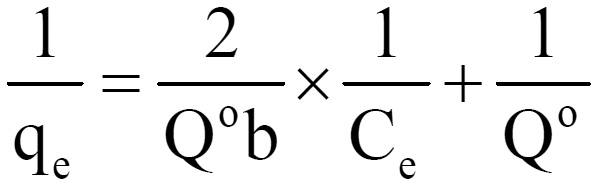
where Q0 and b are Langmuir constants. The plot of 1/Ce Vs 1/qe was found to be linear, indicating the applicability of Langmuir model. The parameters Qo and b have been calculated and presented in Table 1. The Langmuir constant Qo is a measure of adsorption capacity and b is the measure of energy of adsorption. In order to observe whether the adsorption is favorable or not, a dimensionless parameter ‘R’ obtained form Langmuir isotherm is.
R = (1+b× cm)-1
where b is Langmuir constant and Cn is maximum concentration used in the Langmuir isotherm. The adsorption of Lead (II) on coconut shell is a favourable process as “R” values lie between zero to one. Coefficients of co-relation (r) are also shown in Table -1.The applicability of freundlich isotherm was also tried using the following general equation.
qe = k.CeB
linearised form of this equation is
log qe = B.logCe + log k
where B & k are Freundlich constants. These constants represent the adsorption capacity and the adsorption intensity respectively.
Plot of log qe vs log Ce was also found to be linear. The values of B and k are presented in Table 1. Since the values of B are less than 1, it indicates favourable adsorption.
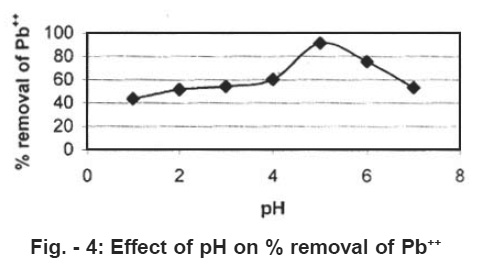 |
Figure - 4: Effect of pH on % removal of Pb++ Click here to view figure |
Results and Discussion
Effect of concentration of metal ion and contact time
The response of Adsorbate does and contact time on the removal of Lead (II) is presented in figure 1. The observation reveal that an increase in the adsorbate dose, rate of adsorption increase upto certain level and then it become constant. Also as the time of contact increase, adsorption increase and then it become constants.
Effect of pH on the removal of Lead (II)
The effect of pH on the removal of Lead II is shown in figure 4. Experiment were conducted at the constant initial Lead (II) concentration, adsorbent dose (coconut shell) of 0.5gm/100 and the contact time of 4hours. The pH of the aquoues solution is an important controlling parameter in the adsorption process. It was observed that the percentage removal of Lead (II) is higher at pH = 5 and then decrease with increase of pH.
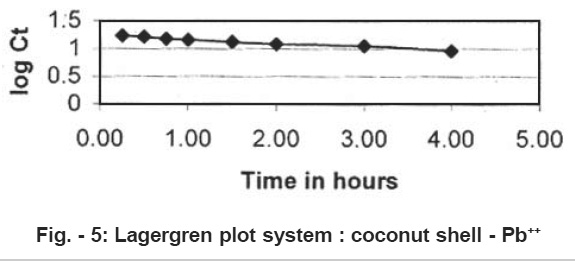 |
Figure - 5: Lagergren plot system : coconut shell - Pb++ Click here to view figure |
Effect of Particle Size
The adsorbent particle size has significant on the kinetics of adsorption. The influence of particle size furnishes important for achieving optimum utilization of adsorbent. Four-particle size 50, 100, 150, 200 micron size (Indian Standard Sieves) under optimum condition. It is found that, as the particle size increase the rate of adsorption decrease.
Kinetic of Adsorption
0.5 gm of coconut shell and 200ml Pb++ solution was taken in 1000ml R.B. and shake vigorously for about hours. After every 15 minutes, 5ml sample of the solution was withdrawn for the first hour and subsequently the interval between the sample withdrawn was increased to 30minutes. The concentration of the metal ions in the sample, withdrawn were determined by the spectrophotometry and were designated as Ct and the value of the concentration of the metal ion on the coconut shell as the same time interval estimated using the relation.
q = (Co – Ct) V/W
The rate of adsorption of Lead (II) on coconut shell was studied by using the first order rate equation proposed by Lagergren.11
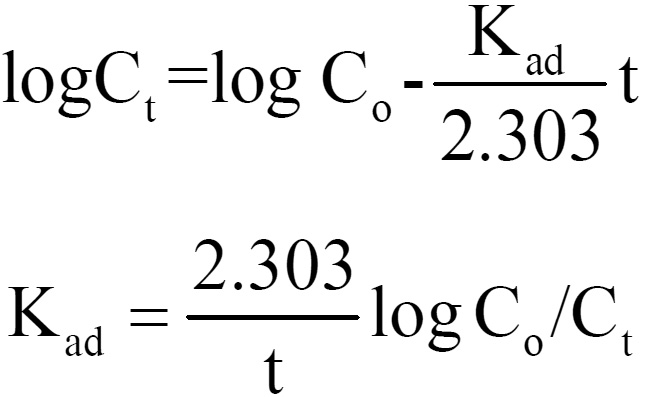
where Kad is the rate constant for adsorption. The plote of log Ct vs t is shown in Fig. 5.
Conclusion
The following conclusion have been drawn from the present study:
- The percentage retrieval of Lead (II) is formed to be increase with decrease the initial concentration of Lead (II). The removal is found rapid in initial stages followed by slow adsorption up to saturation limit.
- The developed technique of retrieval of Lead (II) ions using coconut shell appears to be a cheap and practically viable for the use of semiskilled worker in the villages.
- The present work on adsorption process in good agreement with Langmuir isotherm indicating monolayer adsorption process.
- The result on adsorption process reveals that at pH = 5.0, Lead (II) uptake capacity is better.
- The straight lines plots of log Ct vs t for the adsorption show the validity of Lagergren equation and suggest the first order kinetics.
- Regeneration studies are not necessary with the view that the cost of the adsorbent is very low and it can be disposed of safely.
References
- Mamta Tomer, “Quality Assessment of water and wastewater”, Lewis Publisher Boca Ratan., (1999).
- Sing D.K. and Lal Jyosna, “Removal of toxic heavy metal ion from waste water by coal based adsorbent” Pollution Research., (1992) 11, 37-42.
- Chandra S.V., “Toxic Metal in Environment”, Published by Industrial toxicology research center Lucknow.
- Sohail Ayub and S. Iqbal Ali., “Chromium removal by adsorptions on coconut shell”, IAEB, (2003) 30, 30-36.
- S.B. Shukla., V.D.Sakhardancde. Column studies on metal ions removal by dyed cellulosic. Material J. Appli. Polym Science. (1992) 44(5), 903-10.
- V.K. Lhangan, D.B. Bankar and S.S. Dare effectiveness of terminalia bellerica bark for scavenging zinc ions. Chem Environment Res. (1992) 1(1) 87-94.
- P.R. Rampure and P.V.Patilk. Use of modified Dhoda Bark for scavenging Cd ions from industrial waste-water Jr. of Industrial pollution control (1996) 12(1).
- P.R. Rampure and P.V.Patilk. Use of palsa Bark substrate for the recovery of Cu, Pb, Zn, Ni from waste-water industrial waste-water Jr. of Industrial pollution control (1996) 12(1).
- Sasakio, Akio, Sakamoto, Toshio, Japan, Kokal Tokkyo Koho 8024, 545 21 Feb, CA (1980) 93 : 79281.
- Vogel, A.I “Textbook of quantitative Inorganic Analysis” Fourth Edition, (1978) 735-736.
- Lagergren, s and Bil K, Svenska Vatenska-psakad hand 24(1998).






