The study on the reduction process of Vat dye on cotton fabric using ferrous sulphate and its effect on effluent load
P. Santhi1 * and J. Jeyakodi Moses2
1
Department of Chemistry,
Muthayammal Engineering College,
Rasipuram,
637408
India
2
Department of Chemistry,
PSG College of Technology,
Coimbatore,
641 004
India
DOI: http://dx.doi.org/10.12944/CWE.3.2.05
In textile chemical processing industries vat dye is one of the important dyes for coloration of cotton, when high fastness properties are required. But, vat dye has the problem of insolubility in water .Hence, the process of vatting is needed to reduce and solubilize the dye in water. Vat dye is normally dissolved in water using sodium hydrosulphite (hydrose) as reducing agent and sodium hydroxide as solubilising agent suitable for dyeing. In this work, an attempt is made to reduce the vat dye using ferrous sulphate (2% owm) and its combination with hydrose as ferrous sulphate (1.5% owm) + hydrose (0.5% owm) and ferrous sulphate (1%owm) + hydrose (1%owm).The effect of ferrous sulphate as reducing agent and its combination with hydrose is compared with the conventional reducing agent (hydrose).The effluent load is analysed for all samples and can be compared among themselves. The other test parameters like the strength of the fabric, rubbing fastness, washing fastness, and light fastness, were also studied and compared
Copy the following to cite this article:
Santhi P, Moses J.J. The study on the reduction process of Vat dye on cotton fabric using ferrous sulphate and its effect on effluent load. Curr World Environ 2008;3(2):239-244 DOI:http://dx.doi.org/10.12944/CWE.3.2.05
Copy the following to cite this URL:
Santhi P, Moses J.J. The study on the reduction process of Vat dye on cotton fabric using ferrous sulphate and its effect on effluent load. Curr World Environ 2008;3(2):239-244. Available from: http://www.cwejournal.org/?p=827
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-05-12 |
|---|---|
| Accepted: | 2008-08-17 |
Introduction
Vat dye is one of the important dyes used on the dyeing of cotton textiles due to its overall good fastness properties suitable for the end use products.¹ Vat dye has the problem of insolubility in water, but when reduced with a strong reducing agent like hydrose and solubilising agent sodium hydroxide, it is solubilized. The reduced dyestuff penetrates into the fiber and it is reoxidised on the fiber back to the insoluble form, which remains fixed in the fabric.2-3 The use of sodium hydrosulphite is being criticized for the formation of non-environment friendly decomposition products such as sulphite, sulphate, thiosulphate and toxic sulphur.4 Therefore many attempts are being made to create alternate for the sodium hydrosulphite that cause less pollution.
The replacement of sodium hydrosulphite by organic reducing agents like a-hydroxy ketones5-6 which meets the requirements in terms of reductive efficiency and biodegradability was previously investigated. However, such compounds are expensive and their use is restricted to closed systems due to the formation of strong smelling of condensation products in an alkaline solution. Some other reducing compounds such as hydroxyalkyl sulphonate, thiourea, have also been recommended.7-8 The relatively low sulphur content and lower equivalent mass than that of hydrosulphite lead to lower amounts of sulphur based salt load in the waste water. However, in these cases too, it is not possible to dispense with sulphur based problems totally.
Ferrous hydroxide being a strong reducing agent in alkaline medium has also been explored for reducing organic dye stuffs. The reducing effect of ferrous hydroxide increases with increase in pH. However, ferrous hydroxide is poorly soluble in an alkaline solution and gets precipitated. It has to be complexed in order to hold it in solution.9 Free ferrous hydroxide can be aerated and converted to ferric hydroxide which acts as a flocculent and brings down waste water load. Tartaric acid has also been tried as a ligand by Chakra borty10 for complexing ferrous hydroxide in the presence of sodium hydroxide for reduction and dyeing of cotton with indigo and other vat dyes at room temperature.
The present study is carried out to see the effect of ferrous sulphate as reducing agent on textile material. The results of dyeing using Ferrous sulphate (2% owm), Ferrous sulphate (1.5% owm) combined with hydrose (0.5% owm) and Ferrous sulphate (1% owm) combined with hydrose (1% owm) as reducing agent have been compared with those of the conventional dyeing with hydrose, in terms of tensile strength, rubbing fastness, washing fastness, light fastness and effluent parameters like pH, TDS, Total Alkalinity, Sulphate, Sodium, BOD and COD.
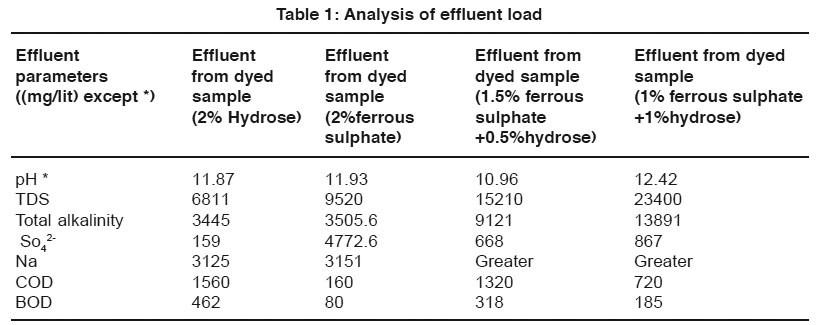 |
Table 1: Analysis of effluent load Click here to view table |
Material
100% cotton poplin fabric was used with following particulars:
Ends / Inch, 132 : Picks / Inch, 70: Warp Count, 37s: Weft Count, 41s
The chemicals like sodium hydrosulphite (AR) and ferrous sulphate (AR) were used as reducing agents; sodium hydroxide (AR), turkey red oil (LR), sodium carbonate (AR), and soap powder (LR) was used as other main chemicals and auxiliaries. The dye selected was commercial Navinon Jade green FFBU/ C (Jade green XBN).
 |
Table 2: Effect of reducing agents on fabric strength Click here to view table |
Methods
Dyeing Procedure for Vat Dyes
The pretreated cotton fabric was vat dyed as per the following
- Conventional Dyeing was carried out using sodium hydrosulphite as reducing agent.9
- Dyeing was carried out similar to the conventional one but with Ferrous Sulphate as reducing agent and Ferrous Sulphate combined with hydrose by the following recipe;
- Vat Dye (Navinon JadegreenXBN) – 2% (owm); T.R.O – 2% (owm); Ferrous sulphate – 2% (owm); NaOH – 2% (owm); M.L.R – 1: 30 Temp – 50– 60; Time – 45 minutes
- Vat Dye (Navinon JadegreenXBN) – 2% (owm); T.R.O – 2% (owm) Ferrous sulphate – 1.5% (owm) + hydrose 0.5% (owm); NaOH – 2% (owm); M.L.R-1: 30; Temp-50-60; Time-45 minutes
- Vat Dye (Navinon JadegreenXBN) – 2% (owm) T.R.O – 2% (owm); ferrous sulphate – 1% (owm) + hydrose 1% (owm) NaOH-2% (owm); M.L.R-1: 30; Temp-50-60; Time-45 minutes
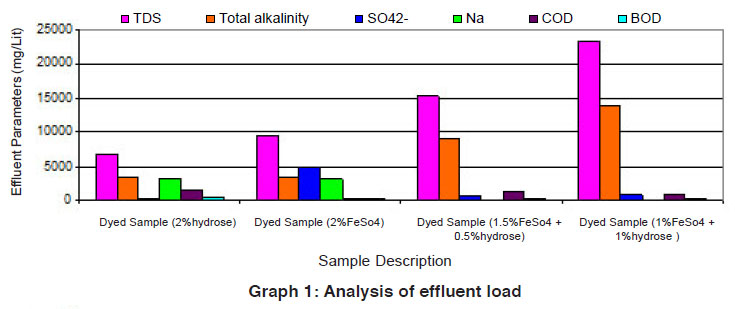 |
Graph 1: Analysis of effluent load Click here to view graph |
Fabric Strength
MAG Electronic Tensile Strength Tester was used to determine the strength of the dyed fabrics with the specimen size of 25mm × 150mm both in warp and weft way by random sampling method.
Colour Fastness to Rubbing, Washing and Light
AATCC standardized crock meter was used to determine the rubbing fastness under wet and dry condition to asses the colour change and staining property. The dyed samples were washed under condition IIIA of the AATCC Test Method 124-2001 to determine the colour change effect of fabrics. Light fastness tests were carried out according to AATCC Test Method 16 E-1998. The samples were exposed to 5, 10 AFUs (AATCC Fading Unit) to determine the colour change.12
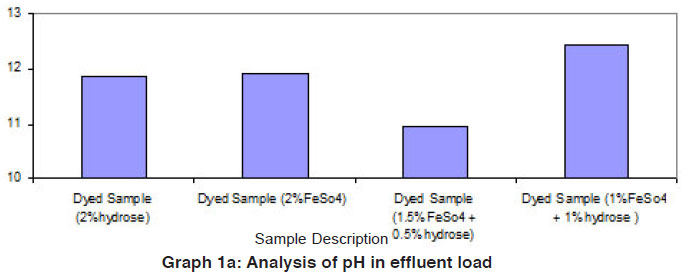 |
Graph 1a: Analysis of pH in effluent load Click here to view graph |
Effluent Load from Dye Effluent
The effluent parameters such as Total Dissolved Solids, total alkalinity, sulphate ion, sodium ion, COD, BOD and pH are tested from the effluent load of the vat dyed baths.
 |
Graph 2: Effect of reducing agents on fabric strength (pretreated samples) Click here to view graph |
Results and Discussion
Effluent from Dye Solution
The data of effluent loads obtained from different dye baths involved for vat dyeing on cotton using the reducing agents such as hydrose 2% owm, ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm are given in Table 1 and shown in Graphs I and Ia. The parameters such as TDS, total alkalinity, sulphate ion, sodium ion, COD, BOD and pH are considered in the effluent load. The parameters TDS, total alkalinity and sodium ion are very high in the effluent of dye baths using different reducing agents (hydrose 2% owm, ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm). The parameter TDS is very high in the dye bath of ferrous sulphate 1% owm + hydrose 1% owm as reducing agent (23400mg/L) compared with other three (hydrose 2% owm à 6811 mg/L and ferrous sulphate 2% owm à 9520 mg/L and ferrous sulphate 1.5% owm + hydrose 0.5% owmα 15210 ). The effluent parameters such as COD and BOD are least in the dye bath using ferrous sulphate 2% owm as the reducing agent. There is no least factor from the effluents of dye bath from ferrous sulphate 1% owm + hydrose 1% owm.The pH is also greater in the effluent from the dyed sample using ferrous sulphate 1% owm + hydrose 1% owm as reducing agent.
 |
Graph 2a: Effect of reducing agents on fabric strength (Dyed samples) Click here to view graph |
Tensile Strength of Cotton Fabric
The tensile strength both in warp direction and in weft direction of cotton fabric in grey form, and after different treatments (scouring, bleaching, and dyeing) is given in Table II and shown in Graph II and Graph IIa. There is a substantial reduction in the tensile strength of cotton fabric both in warp direction and weft direction after bleaching. Due to vat dyeing, the tensile strength of cotton fabric in warp direction is reduced while in weft direction it is increased. The cotton fabric dyed with vat dye using Ferrous sulphate (2% owm) as reducing agent shows good average fabric strength both in warp and weft directions (32.81Kg and 14.75 Kg respectively) compared with vat dyeing using other form of reducing agents (hydrose 2% owm , ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm ). The tensile strength in warp direction is reduced after dyeing using different form of reducing agents (hydrose 2% owm ,ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm) compared with that of grey cotton fabric (33.99 Kg); while it is increased in weft direction after vat dyeing using different form of reducing agents (hydrose 2% owm, ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm) compared with that of grey cotton fabric (13.25 Kg).
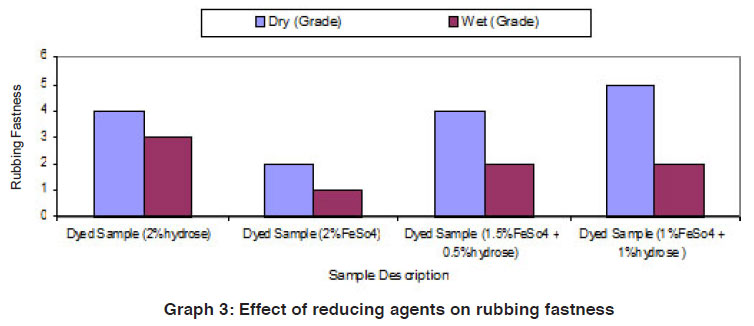 |
Graph 3: Effect of reducing agents on rubbing fastness Click here to view graph |
The percent loss in tensile strength of cotton fabric in warp direction after dyeing using the reducing agents hydrose 2% owm, ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm) is 13.4%, 3.5%, 10.9%and 16.6% respectively, while in the weft direction it is in the increasing trend of 11.7%, 11.3%, 20.1% and 3.47% (loss in tensile strength) respectively. The average tensile strength both in warp direction and in weft direction in the cotton fabric vat dyeing using reducing agents hydrose 2% owm,ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm is 1.7% loss, 7.8% gain , 9.2% gain and 13.1% loss respectively.
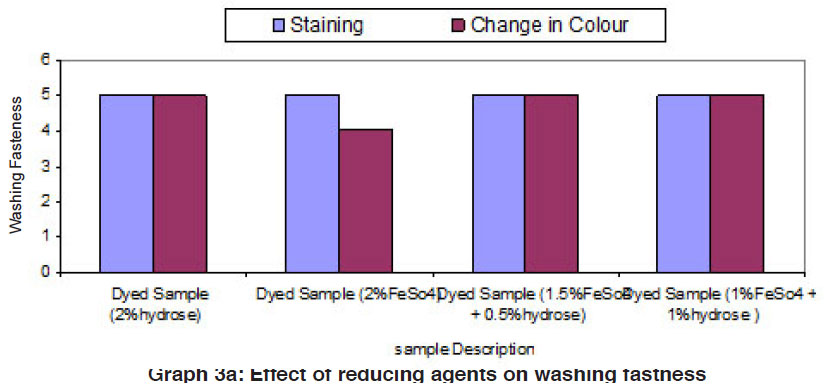 |
Graph 3a: Effect of reducing agents on washing fastness Click here to view graph |
Rubbing Fastness, Washing Fastness and Light Fastness of Vat Dyed Cotton Fabric
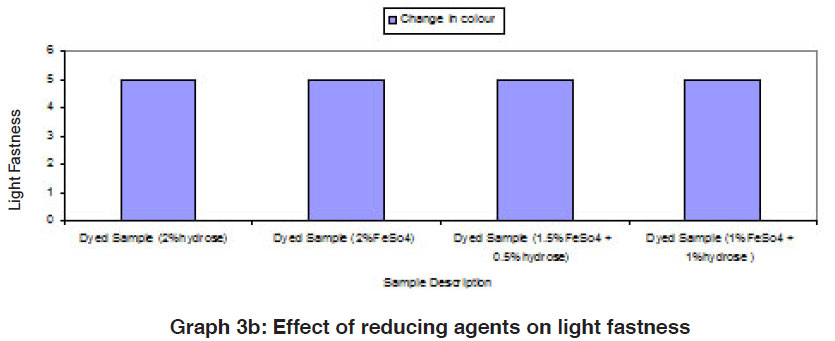
The fastness properties (rubbing fastness, washing fastness and light fastness) of vat dyed cotton fabric using different form of reducing agents (hydrose 2% owm, ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm ) are given in Table III and shown in Graphs III, IIIa and IIIb. There is an overall good fastness properties on the cotton fabric dyed with vat dye using different form of reducing agents such as hydrose 2% owm, ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm . As understood, the rubbing fastness of vat dyed cotton fabric obviously gives good rating in the dry state (range 4) compared to the wet state (range 2 – 1) for all the reducing types involved in vat dyeing except dyed sample (2% ferrous sulphate)both in dry state (2) and in wet state (1). The washing fastness is excellent (rating 5) on the cotton fabric dyed with vat dye using all these reducing agents (hydrose 2% owm, ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm). The light fastness is also good (rating 5) on the cotton fabric dyed using vat dye with the help of different form of reducing agents (hydrose 2% owm, ferrous sulphate 2% owm and ferrous sulphate 1.5% owm + hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm).
 |
Graph 3a: Effect of reducing agents on washing fastness Click here to view graph |
The reducing agent ferrous sulphate (2% owm) and its combination ferrous sulphate 1.5% owm with hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm,also helps to give good fastness properties (Rubbing fastness, washing fastness and light fastness) on cotton fabric dyed with vat dye, similar to the conventional reducing agent, hydrose (2% owm) except rubbing fastness of dyed sample using 2% ferrous sulphate,and its combination (ferrous sulphate1.5% owm with hydrose 0.5% owm and ferrous sulphate 1% owm + hydrose 1% owm) in wet state.
The involvement of ferrous sulphates as reducing agent in vat dyeing is good based on following results:
The average effluent load from the vat dye bath using ferrous sulphates is not more compared to that of hydrose assisted vat dye baths.
The fabric strength of cotton fabric dyed with vat dye using ferrous sulphates as reducing agent is good similar to that of undyed fabric and those of the hydrose assisted dyed fabric.
The fastness properties such as rubbing, washing and light are very good in all the cases of vat dyeing and ferrous sulphates assisted dyeing are not inferior to hydrose assisted vat dyeing.
References
- Philips.D., J.SOC Dyers Colour., (1996) 12: 183.
- Baumgarte.U., Melliand Textilber., (1987) 68: 189-276.
- Baumgarte.U, Rev. Prog. Color., (1987) 17: 29.
- Aspland.J.R. Text Chem. Color., (1992) 22: 24.
- S.Federer-Lerch., Ph. D. Thesis, ETH Zurich, (1995) 11: 351.
- M.Jermini, Ph.D. Thesis, ETH Zurich. (1997) 12: 024.
- Makarov S.V., Russ. Chem. Rev., (2002) 70: 885.
- Marte, Text Praxix int., (1989) 44: 737.
- Somet B., Melliand Textileber., (1995)76: 161.
- Chavan R.B and Chakrabarthy J.N., Indian J. Fibre Text Res., (2000) 5: 130.
- MD Teli, Roshan Paul, Sachin M Landage and Arnab Aich., Indian Journal of Fibre and Textile Research., (2001) 26: 101-107.
- Rekha R. and K.S. Traporewala., Manmade Textiles in India., (April 2002) 127-132.






