Comparative study of Drinking Water Quality Parameters of three Manmade Reservoirs i.e. Kolar, Kaliasote and Kerwa Dam
Ranjeeta Choudhary1 * , Pushpa Rawtani2 and Monika Vishwakarma3
DOI: http://dx.doi.org/10.12944/CWE.6.1.21
A physico-chemical study of the Kerwa dam, Kolar dam and Kaliasote dam of Bhopal has been carried out to examine the suitability of surface water for drinking purposes. Water Samples were collected from two sampling stations of each dam and analyzed (APHA, AWWA, WEF, 1998) for the physico–chemical parameters such as temperature, pH, electrical conductivity, total hardness, calcium hardness, magnesium hardness, sulphate, fluoride and chemical oxygen demand (COD) to know the present status of the water bodies. The concentrations of investigated parameters in the water samples were within the permissible limits of the World Health Organization drinking water quality guidelines except for the values of COD and total hardness. The value of COD was found in the range of 18 mg/l to 30 mg/l which was much higher than the maximum permissible limits as prescribed by WHO standards (1993). The value of total hardness ranges from 118 mg/l to 170 mg/l which is also above the permissible limit as prescribed by WHO standards.
Copy the following to cite this article:
Choudhary R, Rawtani P, Vishwakarma M. Comparative study of Drinking Water Quality Parameters of three Manmade Reservoirs i.e. Kolar, Kaliasote and Kerwa Dam. Curr World Environ 2011:6(1);145-149 DOI:http://dx.doi.org/10.12944/CWE.6.1.21
Copy the following to cite this URL:
Choudhary R, Rawtani P, Vishwakarma M. Comparative study of Drinking Water Quality Parameters of three Manmade Reservoirs i.e. Kolar, Kaliasote and Kerwa Dam. Curr World Environ 2011:6(1);145-149. Available from: http://www.cwejournal.org/?p=1305
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-01-11 |
|---|---|
| Accepted: | 2011-02-18 |
Introduction
The Kerwa, Kolar and Kaliasote dam are the three main dams of Bhopal city as they are used for various activities like drinking, irrigation, recreational etc. Kolar dam (latitude 22° 57' 37" and longitude 77° 20' 24") is a major masonry dam which is located about 32 km from Bhopal near Lawakhedi village in Sehore District. It is constructed across the Kolar River near Birpur, a tributary of Narmada. The dam is about 45 m high. This reservoir has a catchment area of 508 km2. The gross storage capacity is 270 M cum and live storage capacity is 265 M cum Kaliasote dam (latitude 25° 11' 45' N and longitude 77° 24' E) was constructed near village Chuna Bhatti across the river Kaliasote, a tributary of Betwa River in Huzur Tehsil of Bhopal District. The Kaliasote dam is 1080 m long. Its height is 34.25 m and has gross storage capacity of 35.387 M cum. The Dam is used for irrigation and recreational purposes. Kerwa dam is situated in the subtropical region of the Bhopal District. Kerwa Dam was constructed on Kerwa River near Bhopal city. This reservoir has a catchment area of 34.50 km2 and its gross storage capacity is 25 M cum. The water of this dam is used for multipurpose activities like drinking, irrigation, fish culture etc. Continuous expansion of Bhopal city in terms of area and population has negative impacts on these dams. These dams are loosing their beauty and identity because of pollution and encroachment.
Material and Methods
For the present investigations the Kerwa dam, Kolar dam and Kaliasote dam of Bhopal (M.P) were selected. Two sampling stations were chosen from each dam and surface water samples were collected from all the six sampling stations (namely S1, S2, T1, T2 and U1, U2) in the month of June 2010. The sampling station S1, T1 and U1 belongs to coastal region where as S2, T2 and U2 belongs to centre region of water bodies. Each water sample was analyzed for nine parameters such as temperature, pH, electrical conductivity, total hardness, calcium hardness, magnesium hardness, sulphate, fluoride and chemical oxygen demand by standard methods prescribed by APHA, AWWA, WEF (1998) and NEERI (1991).
Result and Discussion
The data revealed that there were considerable variations in the examined samples. The results of analysis of various physico-chemical parameters of water of Kerwa dam, Kolar dam and Kaliasote dam was summarized in table 1. A comparison of physico-chemical characteristics of the studied water samples has also been made with WHO standards (1993) and BIS standards (1991). These parameters are discussed below:
Temperature
Temperature was in the range of 25.2o C to 32o. Temperature is one of the most important factors in aquatic environment (Singh et al, 2005). Temperature also affects solubility of oxygen in water. Solubility of oxygen in water increases with decreasing temperature (Joshi et al, 2001). Water temperatures fluctuate naturally both daily and seasonally. Aquatic organisms often have narrow temperature tolerances. Thus, although water bodies have the ability to buffer against atmospheric temperature extremes, even moderate changes in water temperatures can have serious impacts on aquatic life, including bacteria, algae, invertebrates and fish.
Hydrogen Ion concentration (pH)
pH indicates the intensity of acidic or basic character at a given temperature. Measurement of pH is one of the most important and most frequently used tests in determining water quality. Every phase of water treatment and water supply like acid-base neutralization, water softening, precipitation, coagulation, disinfection, corrosion control etc is pH dependent. The pH of the water samples were found in the range of 7.40 to 8.20 i.e. slightly alkaline. The maximum value of pH was recorded at sampling station U2 of Kaliasote dam which is 8.20 and the minimum value of pH was recorded at sampling station T1 of Kolar dam which is 7.40. The maximum permissible limit of pH as prescribed by WHO is 7.0 to 8.50. All the reservoirs have pH values within the desirable and suitable range.
Electrical Conductivity
It is a measure of the ability of an aqueous solution to carry an electric current. It depends on the presence of ions, on their total concentration, mobility and temperature of measurement. Higher value of conductivity shows higher concentration of dissolved ions. Conductivity of water sample was found in the range of 222-385 micromhos per cm2, which is much below the WHO standards. Electrical conductivity is considered to be a rapid and good measure of dissolved solids. Conductivity is an important criterion in determining the suitability of water for irrigation. The maximum value of electrical conductivity (385 mg/l) was found at U2 sampling station of Kaliasote dam followed by U1 sampling station of same dam. The high value of conductivity was due to presence of dissolved salts. The least value (222 mg/l) was recorded at S1 sampling station of Kerwa dam. The value of electrical conductivity follows the order Kerwa dam < Kolar dam < Kaliasote dam.
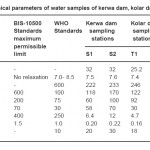 |
Table 1: Physico-chemical parameters of water samples of kerwa dam, kolar dam and kaliasote dam Click here to View table |
Total Hardness
The hardness of water is not pollution parameter but indicates water quality mainly in terms of Ca2+ and Mg2+ expressed as CaCO3(De, A.K, 2006). The total hardness was found to be in the range of 118 mg/l to 170 mg/l. It was found to be 170 mg/l at S2 sampling station of Kerwa dam followed by T2 sampling station of Kolar dam. The least value (118 mg/l) was recorded for S1 sampling station of Kerwa dam. Thus the lowest and highest values of total hardness were observed at Kerwa dam. It is much higher than the permissible limit as prescribed by WHO but well within the permissible limits as prescribed by BIS standards. The total hardness is mainly due to presence calcium and magnesium ion. The water containing excess hardness is not desirable for potable water. It consumes more soap during washing of clothes. As per Moyle et al (1956) all value above 64 mg/l comes under the category of hard water.
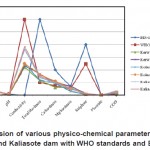 |
Fig. 1: Comparision of various physico-chemical parameters of Kerwa dam, Kolar dam and Kaliasote dam with WHO standards and BIS standards Click here to View figure |
Calcium Hardness
The main sources of calcium in natural water are various types of rocks, industrial waste and sewage. There is evidence that hard water plays a role in heart diseases (Sastry et al 1998). Its value was found in the range of 60 mg/l to 112 mg/ l which is slightly higher than the permissible limit as prescribed by WHO but well within the permissible limits as prescribed by BIS standards. T2 sampling station of Kolar dam has highest value of calcium hardness i.e. 112 mg/l followed by S2 sampling station of Kerwa dam. The minimum value (60 mg/l) of calcium hardness was observed at S1 sampling station of Kerwa dam.
Magnesium Hardness
Its value was found in the range of 30 mg/ l to 70 mg/l. Its value is slightly higher than the permissible limits as prescribed by WHO but well within the permissible limits as prescribed by BIS standards. The maximum value of magnesium hardness (70 mg/l) was found at S2 sampling station of Kerwa dam. The high value of magnesium hardness was due to human settlement around the dam. The least value (30 mg/l) was recorded at T1 sampling station of Kolar dam. Higher concentration of magnesium makes the water unpalatable and act as laxative to human beings.
Fluoride
The value of fluoride was found in the range of 0.20 mg/l to 0.38 mg/l. The maximum value (0.38 mg/l) and minimum value (0.20 mg/l) of fluoride was recorded at U2 and T1 sampling station of Kaliasote and Kolar dam respectively. Its value is within the permissible limit as prescribed by WHO.A fluoride concentration of approximately 1.0 mg/l in drinking water effectively reduces dental caries with out harmful effects on the health. Fluoride may occur naturally in water or it may be added in controlled amounts. Some fluorosis may occur when the fluoride level exceeds the recommended limits.
Sulphate
It occurs in natural water in concentration ranging from a few to several thousand milligrams per litre. Mine drainage wastes may contribute high sulphate by virtue of pyrite oxidation. Excess sodium sulphate should not be present in drinking water as they cause cathartic action. The value of sulphate was found in the range of 4.7 mg/l to 13.2 mg/l. Its value is much lower than the permissible limit as prescribed by WHO and BIS standards. Its value follows the order Kolar dam (4.70 mg/l & 5.80 mg/l) < Kerwa dam (6.40 mg/l & 12 mg/l) < Kaliasote dam (12.80 mg/l & 13.20 mg/l). High concentration of Sodium and magnesium sulphate is associated with respiratory illness.
Chemical Oxygen Demand (COD)
Chemical oxygen demand is a valuable water quality parameter. COD is a measure of the oxygen equivalent of the organic matter in a water sample that is susceptible to oxidation by a strong chemical oxidant, such as dichromate. It is an index of organic content of water because the most common substance oxidized by dissolve oxygen in water is organic matter having biological origin i.e. dead plant and animal wastes (Singh,A, 2006). COD values convey the amount of dissolved oxidisable organic matter including the non-biodegradable matters present in it. The value of COD was found in the range of 18 mg/l to 30 mg/l. Its value is higher than the permissible limit prescribed by WHO. The increase in COD concentration was found in the bottom water where organic matter is more (Prasad & Qayyum, 1976).
Conclusion
The present study leads to following conclusions:
- Data reveals that all the three dams are polluted to some extent but Kerwa dam is most polluted as indicated by a very high value of COD. The main sources of pollution of Kerwa dam are the human settlement around the dam and their activities.
- Data also indicates that in all the three water bodies total hardness, calcium hardness and magnesium hardness were found to be beyond the permissible limits as prescribed by WHO but well within the permissible limits as prescribed by BIS standards.
- Data also reveals that in all the three dams the values of parameters such as pH, electrical conductivity, sulphate and fluoride were well within the WHO and BIS permissible limits for drinking water.
- To improve the quality of water of all the three dams there should be continuous monitoring of the pollution level.
References
- APHA, AWWA, WEF, Standard methods for the examination of water and waste water (20th edn.) Washington, DC: American Public Health Association (1998).
- B.I.S. Bureau of Indian Standards Drinking water specification, Ist revision, ISS 10500 (1991).
- De, A. K., Environmental chemistry (6th edn.). New Delhi, India: New Age International Publishers (232) (2006).
- Joshi, P.C and Singh, Analysis of certain physico-chemical parameters and planktons of fresh water hill stream at Nanda Devi biosphere reserve. Uttar Pradesh J. Zoo., 21: 177-179 (2001).
- Moyle, J., Relationship between the chemistry and Minnesota surface waters and wild life management. J. Wild L. Marg, 20:303-320 (1956).
- NEERI (1991). Manual on water and waste water analysis. National Environmental Engineering Research Institute, Nagpur.
- Prasad D.Y. & Qayyum, M.A., Pollution aspects of Upper Lake Bhopal. Indian Journal of Zoology 4(1): 35-46 (1976).
- Sastry, K. V. and Prathima Rathee, Physico-chemical and microbiological characteristics of water of village Kanneli (distt. Rohtak), Haryana. Proc. Acad. Biol., 7(1): 103-108 (1998).
- Singh, A, Environmental chemistry (Ist edn.).Campus books international, Delhi (2006).
- Singh, R.P.and Mathur, P., investigation of variations in physico chemical characteristics of fresh water reservoir of Ajmer city, Rajasthan, Ind. J. Env., 9: 57-61 (2005).
- World Health Organization, Guidelines for drinking water quality-I, Recommendations, 2nd Edi. Geneva WHO (1993).







